






|
Leeper Group | ||||
| | |||||

|

|
 |
 |
 |
|
  | ||||||
|
Abstract 64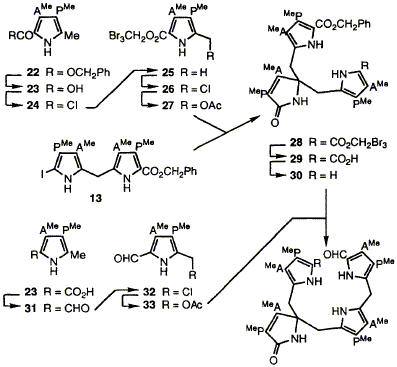 W. M. Stark, C. J. Hawker, G. J. Hart, A. Philippides, P. M. Petersen, J. D. Lewis, F. J. Leeper and A. R. Battersby
W. M. Stark, C. J. Hawker, G. J. Hart, A. Philippides, P. M. Petersen, J. D. Lewis, F. J. Leeper and A. R. Battersby "Biosynthesis of Porphyrins and Related Macrocycles, Part 40. Synthesis of a Spiro-Lactam Related to the Proposed Spiro-Intermediate for Porphyrin Biosynthesis: Inhibition of Cosynthetase" J. Chem. Soc., Perkin Trans. 1,1993, 2875-2892. Full Text Routes are developed for synthesis of the tripyrrolic macrocyclic spiro-lactam 39. A minor product from the synthesis, thought earlier to be an atropisomer, has been shown by molecular mechanics calculations and re-investigation to be a dimer. The octa-acid derived from 39 closely resembles the spiro-pyrrolenine proposed as a biosynthetic intermediate for uroporphyrinogen III. This octa-acid acts as a strong inhibitor of cosynthetase (uroporphyrinogen III synthase) whilst other similar systems which lack some of its functionality do not. These results strongly support the view that the spiro system is indeed the biosynthetic intermediate for formation of uroporphyrinogen III from hydroxymethylbilane.
|
||||||
  | ||||||