






|
Leeper Group | ||||
| | |||||

|

|
 |
 |
 |
|
  | ||||||
|
Abstract 65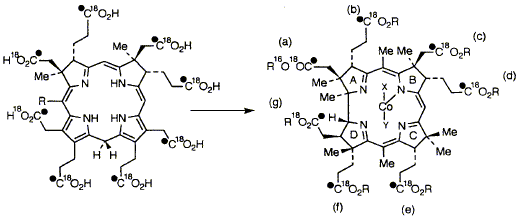 R. A. Vishwakarma, S. Balachandran, A. I. D. Alanine, N. P. J. Stamford, F. Kiuchi, F. J. Leeper and A. R. Battersby
R. A. Vishwakarma, S. Balachandran, A. I. D. Alanine, N. P. J. Stamford, F. Kiuchi, F. J. Leeper and A. R. Battersby "Biosynthesis of Porphyrins and Related Macrocycles. Part 41. Fate of Oxygen Atoms as Precorrin-2 Carrying Eight Labelled Carboxyl Groups (13C18O2H) is Enzymatically Converted into Cobyrinic Acid" J. Chem. Soc., Perkin Trans. 1,1993, 2893-2899. Full Text 5-Amino[1,4-13C2]laevulinic acid and 5-amino[1-13C]laevulinic acid are synthesised and all three 16O atoms of the latter are exchanged for 18O. The 13C,18O-labelled material is then converted in vitro into precorrin-2 by the combined action of four genetically overproduced enzymes. The product is isolated in its aromatised form, sirohydrochlorin and 13C-NMR shows that all 8 carboxyl groups retain both oxygen atoms throughout the biosynthesis. A cell-free enzyme preparation from Propionibacterium shermanii converts the 13C,18O-labelled sirohydrochlorin via precorrin-2 into cobyrinic acid, a late precursor of vitamin B12. 13C-NMR proves that 6 carboxyl groups of cobyrinic acid (b-g, inclusive) retain both oxygen atoms whereas the a-carboxyl group undergoes specific loss of one labelled oxygen atom.
|
||||||
  | ||||||