






|
Leeper Group | ||||
| | |||||

|

|
 |
 |
 |
|
  | ||||||
|
Abstract 40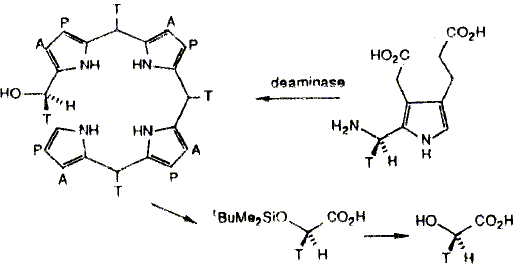 F. J. Leeper and A. R. Battersby
F. J. Leeper and A. R. Battersby "Chirally Tritiated and Deuteriated Compounds in Studies of Biosynthesis" in Synthesis and Applications of Isotopically Labelled Compounds (Proceedings of the Third International Symposium). 1989, Elsevier, p. 41-46. Porphobilinogen (PBG) deaminase catalyses the tetramerization of the monopyrrole PBG to give a linear tetrapyrrole, hydroxymethylbilane (HMB). The stereochemistry of some of the steps in this reaction has been investigated using PBG chirally labelled with deuterium or tritium at the aminomethyl group (C-11). Two different syntheses of the chirally labelled PBG are described and methods for the analysis of the stereochemistry of the products are detailed. These studies showed that the formation of HMB proceeds with overall retention of configuration at the hydroxymethyl carbon. This rules out the release of the azafuIvene intermediate from the enzyme.
|
||||||
  | ||||||