






|
Leeper Group | ||||
| | |||||

|

|
 |
 |
 |
|
  | ||||||
|
Abstract 41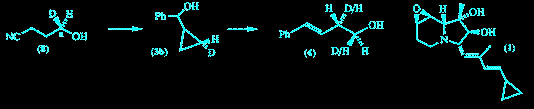
F. J. Leeper and P. Padmanabhan "Stereospecific Nucleophilic Ring-Opening of a Deuteriated Cyclopropylcarbinol" Tetrahedron Lett.,1989, 30, 5017-5020. Full Text The stereospecifically deuteriated phenyl cyclopropyl carbinol (3b) was synthesized from chiral alcohol (8). Ring-opening to the homoallylic alcohol (4) was shown to proceed with clean inversion of configuration by 1H n.m.r. spectroscopy of the ring-opened product with a chiral shift reagent. The indolizidine alkaloid (1) can be degraded to (3) and this will provide a method to analyse the stereochemistry of its biosynthesis.
|
||||||
  | ||||||