






|
Leeper Group | ||||
| | |||||

|

|
 |
 |
 |
|
  | ||||||
|
Abstract 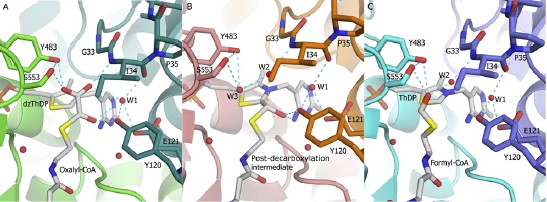 114 114Catrine L. Berthold, Cory G. Toyota, Patricia Moussatche, Martin D. Wood, Finian J. Leeper, Nigel G. J. Richards, Ylva Lindqvist, "Crystallographic snapshots of oxalyl-CoA decarboxylase give new insights into catalysis by non-oxidative ThDP-dependent decarboxylases." Structure, 2007, 15 (7), 853-861. Full Text Despite more than five decades of extensive studies of thiamin diphosphate (ThDP) enzymes, there remain many uncertainties as to how these enzymes achieve their rate enhancements. Here, we present a clear picture of catalysis for the simple nonoxidative decarboxylase, oxalyl-coenzyme A (CoA) decarboxylase, based on crystallographic snapshots along the catalytic cycle and kinetic data on active site mutants. First, we provide crystallographic evidence that, upon binding of oxalyl-CoA, the C-terminal 13 residues fold over the substrate, aligning the substrate a-carbon for attack by the ThDP-C2 atom. The second structure presented shows a covalent reaction intermediate after decarboxylation, interpreted as being nonplanar. Finally, the structure of a product complex is presented. In accordance with mutagenesis data, no side chains of the enzyme are implied to directly participate in proton transfer except the glutamic acid (Glu-56), which promotes formation of the 1',4'-iminopyrimidine tautomer of ThDP needed for activation.
|
||||||
  | ||||||