






|
Leeper Group | ||||
| | |||||

|

|
 |
 |
 |
|
  | ||||||
|
Abstract 31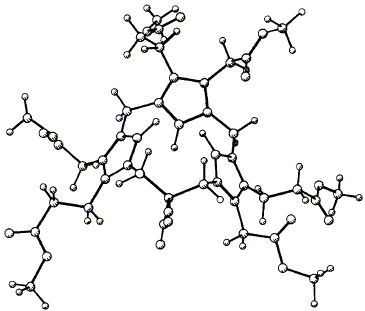
W. M. Stark, M. G. Baker, F. J. Leeper, P. R. Raithby and A. R. Battersby "Biosynthesis of Porphyrins and Related Macrocycles, Part 30. Synthesis of the Macrocycle of the Spiro System Proposed as an Intermediate Generated by Cosynthetase" J. Chem. Soc., Perkin Trans. 1,1988, 1187-1201. Full Text Syntheses are described of two compounds having the 1,4-(propane-1,3-diyl)tripyrrin macrocycle (5), which is the basis of the spiro compound (2) proposed as an intermediate during the cyclization of hydroxymethylbilane (1) to uro’gen Ill (3), catalysed by the enzyme cosynthetase. The first synthesis, of the dinitrile (41), starts with the alkylation of malononitrile with two different chloromethylpyrroles, whereas the second synthesis, of the spiro lactam (59) uses the condensation of nitroalkanes with pyrrole aldehydes. In both cases the macrocycle is completed by addition of a third pyrrole ring followed by acid-catalysed cyclization from an a-free pyrrole onto a hydroxymethyl substituent. The crystal structure of the dinitrile (41) shows a rigid, highly puckered macrocycle and the same conformation in the spiro lactam (59) is used to explain the existence of two non-interconvertible isomers, (61) and (62).
|
||||||
  | ||||||