

10 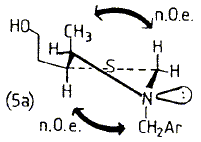
F. J. Leeper and P. N. Lowe
"The Stereochemistry and Conformation of the Diastereomers of Tetrahydrothiamin"
Heterocycles,1983, 20, 65-68.
Full Text
The major isomer of tetrahydrothiamin produced by NaBH4 reduction of thiamin is shown by 1H n.m.r. to be cis (5) and its conformation is predominantly (5a) which should be a major factor in the strong inhibition shown by its pyrophosphate (2) towards the pyruvate dehydrogenase complex af E.coli.
.
.
.
.
.
.
.
.
.
.
.
.
11 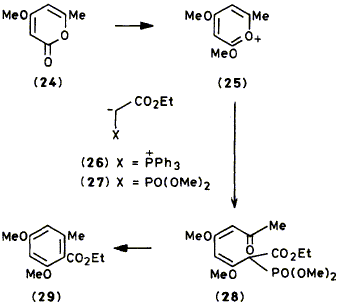
D. A. Griffin, F. J. Leeper and J. Staunton
"Biomimetic Syntheses of Polyketide Aromatics from Pyrylium Salts"
J. Chem. Soc., Perkin Trans. 1,1984, 1035-1042.
Full Text
Following attack by nucleophiles, various methoxypyrylium compounds derived from 2H-pyran-2-ones and 4H-pyran-4-ones react either to produce a linear b-polycarbonyl derivative in which one or more carbonyl groups is derivatised in the form of an enol ether or to form a methylene-2H-pyran. The linear polycarbonyl derivatives can undergo biomimetic cyclisation to generate polyketide aromatic systems; syntheses of the following benzene derivatives are described: ethyl 2,4-dimethoxy-6-methylbenzoate (29), 2,4-dimethoxy-6-methylbiphenyl (31), and 6-(2,4-dimethoxy-6-methylphenyl)-4-methoxy-2H-pyran-2-one (34).
.
.
.
.
.
.
.
.
.
.
.
.
12 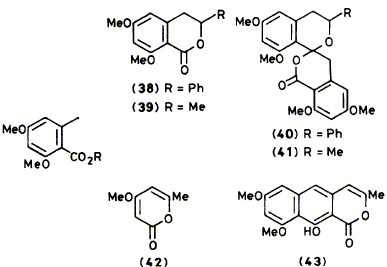
T. A. Carpenter, G. E. Evans, F. J. Leeper, J. Staunton and M. R. Wilkinson
"Reactions of the Carbanion from an Orsellinate Derivative with Electrophiles"
J. Chem. Soc., Perkin Trans. 1,1984, 1043-1051.
Full Text
Esters of orsellinic acid dimethyl ether (11) are attacked by alkyl-lithium reagents to give ketones and in some cases smaller amounts of alkenes as a result of a second nucleophilic attack. However at -78 oC attack on (11) by its own anion (10) is sufficiently slow that the anion can be generated almost quantitatively by lithium di-isopropylamide. Reactions of the anion (10) with various simple electrophiles (D2O, CO2, Mel, Me3SiCI, and Me3SnCI) give the expected products of nucleophilic addition. With aldehydes (PhCHO and MeCHO) the initially formed alcohol lactonizes to give a dihydroisocoumarin. The anion was acylated with acetyl chloride to give the ketone (18). The reactions of the trimethylsilyl derivative (14) are in most cases very similar to the parent compound (11) but the trimethylstannyl derivative (15) regenerates the anion (10) on treatment with MeLi or BunLi. Finally, reaction of the anion (10) with a-pyrone (42) unexpectedly gave the naphthopyrone (43).
.
.
.
.
.
.
.
.
.
.
.
.
13 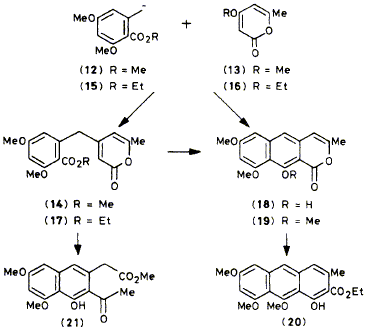
F. J. Leeper and J. Staunton
"Biomimetic Syntheses of Polyketide Aromatics from Reaction of an Orsellinate Anion with Pyrones and a Pyrylium Salt"
J. Chem. Soc., Perkin Trans. 1,1984, 1053-1059.
Full Text
Orsellinate anion (12) shows highly regioselective attack on pyrones (13), (22), and (30),and the products were simply converted into derivatives of the polyketides: toralactone (19), 6-hydroxymusizin (26), and eleutherinol (32); although reaction of the anion (12) with the pyrylium salt (33) is less selective, the major product (34) can give derivatives of either alternariol (36) or rubrofusarin (37) in regiospecific biomimetic reactions.
.
.
.
.
.
.
.
.
.
.
.
.
14 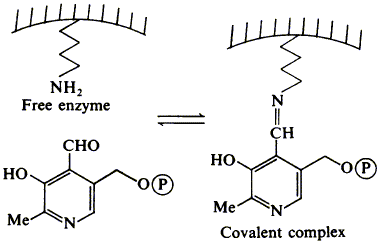
G. J. Hart, F. J. Leeper and A. R. Battersby
"Modification of hydroxymethylbilane synthase (porphobilinogen deaminase) by pyridoxal 5-phosphate"
Biochem. J., 1984, 222, 93-102.
Abstract,
Full Text
When hydroxymethylbilane synthase (porphobilinogen deaminase) from Euglena gracilis is incubated with pyridoxal 5'-phosphate at pH 7.0 and 0 degree C, it rapidly loses part of its activity. The proportion of activity that remains decreases as the concentration of the modifier increases up to approx. 2 mM, above which no further significant inactivation occurs. Dialysis of the partly inactivated enzyme restores its activity, whereas reduction with NaBH4 makes the inactivation permanent. The maximum inactivation achievable from one cycle of the treatment with pyridoxal 5'-phosphate, then with borohydride, is 53 +/- 5%; taking this modified enzyme through second and third cycles causes further loss of activity. The enzyme from Rhodopseudomonas spheroides behaves similarly, but there are quantitative differences. Spectroscopic evidence indicates that the inactivation procedure modifies lysine residues, and labelling studies show that epsilon-N-pyridoxyl-L-lysine is a product when permanently inactivated enzyme is completely hydrolysed. Several lysine residues per molecule of the E. gracilis enzyme are modified by the treatment with pyridoxal 5'-phosphate and borohydride, but only one appears to be essential for enzymic activity, since porphobilinogen protects the enzyme against inactivation and then one fewer lysine residue per molecule of enzyme is affected. It is suggested that, during the biosynthesis of hydroxymethylbilane, the first porphobilinogen unit is covalently bound to the enzyme through the epsilon-amino group of the essential lysine.
.
.
.
.
.
.
.
.
.
.
.
.
15 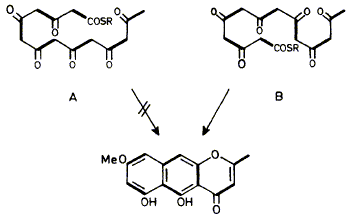
F. J. Leeper and J. Staunton
"The Biosynthesis of Rubrofusarin, a Polyketide Naphthopyrone from Fusarium culmorum: 13C NMR Assignments and Incorporation of 13C and 2H-Labelled Acetates"
J. Chem. Soc., Perkin Trans. 1,1984, 2919-2925.
Full Text
The polyketide chain in rubrofusarin biosynthesis has been shown to adopt the folding pattern B by the incorporation of 13CH313CO2Na and of CD313CO2Na, with observation of the b-isotopic shifts in the 13C n.m.r. spectrum, in the rubrofusarin derivative (5). The extent of retention of deuterium at the various sites is interpreted in terms of an unusual sequence of biosynthetic steps. The assignment of the 13C n.m.r. spectrum of rubrofusarin dimethyl ether (2) was made with the aid of specific deuteriation and 1H-13C n.O.e.'s as well as more standard techniques.
.
.
.
.
.
.
.
.
.
.
.
.
16 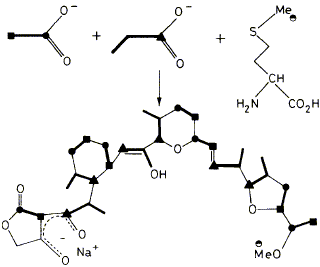
J. M. Bulsing, E. D. Laue, F. J. Leeper, J. Staunton, D. H. Davies, G. A. F. Ritchie, A. Davies, A. B. Davies and R. P. Mabelis
"Biosynthesis of the Polyketide Antibiotic ICI 139603 in Streptomyces longisporoflavus: Assignment of the 13C NMR by Two-Dimensional Methods and Determination of the Origin of the Carbon Atoms"
J. Chem. Soc., Chem. Commun.,1984, 1301-1302.
Full Text
The carbon skeleton of the polyether antibiotic lCI139603 (1) is shown to consist of a polyketide chain, derived from seven acetate units and six propionate units, combined with a C2-unit of unknown origin.
.
.
.
.
.
.
.
.
.
.
.
.
17 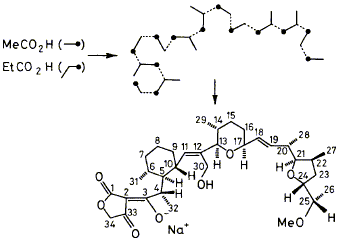
D. M. Doddrell, E. D. Laue, F. J. Leeper, J. Staunton, A. Davies, A. B. Davies and G. A. F. Ritchie
"Biosynthesis of the Polyether Antibiotic ICI 139603 in Streptomyces longisporoflavus: Investigation of Deuterium Retention after Incorporation of CD313CO2H, 13CD3CO2H, and CH3CD213CO2H using 2H NMR and edited 13C NMR Spectra"
J. Chem. Soc., Chem. Commun.,1984, 1302-1304.
Full Text
The mechanism of carbocyclic ring formation in the biosynthesis of the polyether antibiotic ICI139603 (2) was investigated by deuterium retention studies after incorporation of CD313CO2H, 13CD3CO2H, and CH3CD213CO2H using 2H n.m.r. spectroscopy, a- and b-isotopic shifts, and edited 13C n.m.r. spectroscopy.
.
.
.
.
.
.
.
.
.
.
.
.
18 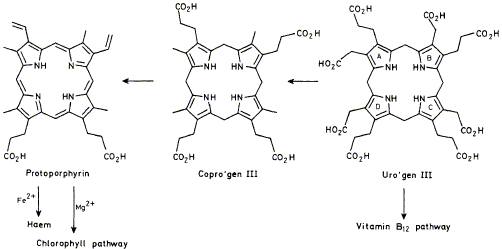
F. J. Leeper
"The Biosynthesis of Porphyrins, Chlorophylls, and Vitamin B12"
Natural Products Reports,1985, 2, 19-47.
Full Text
Reviewing the literature between December 1977 and December 1983.
.
.
.
.
.
.
.
.
.
.
.
.
19 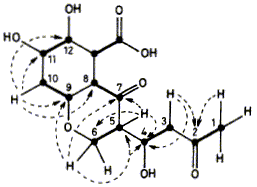 A. K. Demetriadou, E. D. Laue, F. J. Leeper and J. Staunton
A. K. Demetriadou, E. D. Laue, F. J. Leeper and J. Staunton
"The Structure of Polivione, a Polyketide Co-metabolite of Citromycetin in Penicillium frequentans"
J. Chem. Soc., Chem. Commun., 1985, 762-764.
Full Text
Polivione, a polyketide metabolite and probably a precursor of citromycetin, has been isolated from Penicillium frequentans.
.
.
.
.
.
.
.
--- Previous ten abstracts ------ Next ten abstracts ---
.