

.
.
.
.
.
20 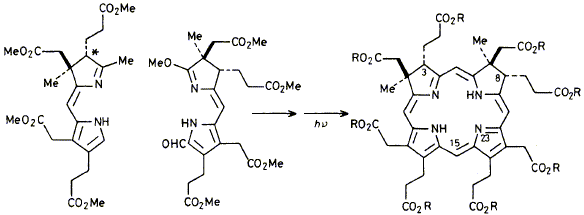
M. H. Block, S. C. Zimmerman, G. B. Henderson, S. P. D. Turner, S. W. Westwood, F. J. Leeper and A. R. Battersby
"Syntheses Relevant to Vitamin B12 Biosynthesis: Synthesis of Sirohydrochlorin and of its Octamethyl Ester"
J. Chem. Soc., Chem. Commun., 1985, 1061-1063.
Full Text
Sirohydrochlorin is an isobacteriochlorin isolated (a) as the metal-free prosthetic group of sulphite reductase and (b) as the aromatised form of the di-C-methylated intermediate on the biosynthetic pathway to vitamin B12; the synthesis is described of the natural enantiomer of sirohydrochlorin and also of its octamethyl ester using a mild photochemical route.
.
.
.
.
.
.
.
.
.
.
.
.
21 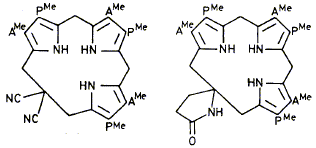
W. M. Stark, M. G. Baker, P. R. Raithby, F. J. Leeper and A. R. Battersby
"The Spiro-Intermediate Proposed for Biosynthesis of the Natural Porphyrins: Synthesis and Properties of its Macrocycle"
J. Chem. Soc., Chem. Commun., 1985, 1294-1296.
Full Text
Two structures have been synthesised containing the novel macrocycle on which is based the hypothetical spiro-intermediate for biosynthesis of the natural porphyrins; the strongly puckered conformation of the macrocycle is shown by X-ray structure analysis.
.
.
.
.
.
.
.
.
.
.
.
.
22 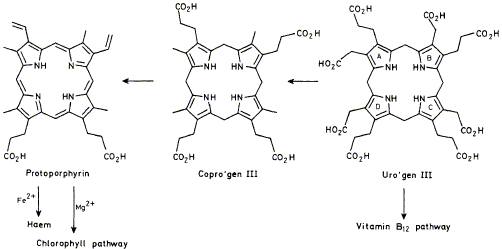
F. J. Leeper
"The Biosynthesis of Porphyrins, Chlorophylls, and Vitamin B12"
Natural Products Reports,1985, 2, 561-580.
Full Text
Reviewing the literature published during 1984.
.
.
.
.
.
.
.
.
.
.
.
.
23 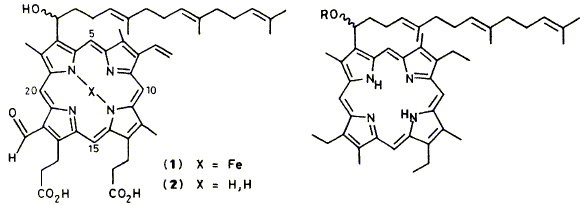
A. R. Battersby, K. S. Cardwell and F. J. Leeper
"Stereochemical Studies on Porphyrin a: Assignment of the Absolute Configuration of a Model Porphyrin by Degradation"
J. Chem. Soc., Perkin Trans. 1,1986, 1565-1580.
Full Text
The synthesis is described of a porphyrin alcohol (22) which has a structure very similar to that of porphyrin a (2). The model porphyrin was resolved by separation of its camphanate esters. Ozonolysis of the 2-nitrobenzoate of each enantiomer in tritiated form gave a derivative of 2- hydroxypentanedioic acid whose configuration was determined by dilution analysis. It is demonstrated that correlation of the stereochemistry of porphyrin a with that of the model (22) will be possible by means of the 1H and 19F n.m.r. spectra of the corresponding esters with (-)-3,3,3-trifluoro-2-methoxy-2-phenylpropanoic acid.
.
.
.
.
.
.
.
.
.
.
.
.
24 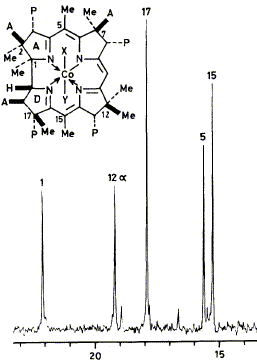
H. C. Uzar, A. R. Battersby, T. A. Carpenter and F. J. Leeper
"Biosynthesis of Porphyrins and Related Macrocycles, Part 28. Development of a Pulse Labelling Method to Determine the C-Methylation Sequence for Vitamin B12"
J. Chem. Soc., Perkin Trans. 1, 1987, 1689-1696.
Full Text
The biosynthesis of vitamin B12 involves enzymic C-methylation of uroporphyrinogen-lII at 8 sites.
Earlier work had established that the first 3 methyl groups are introduced at positions 2,7, and 20 in that
order. Pulse-labelling experiments with 13C-labelled and unlabelled S-adenosylmethionine show that
the remaining 5 C-methyl groups are added in the order 17, 12, 1, 15, 5. The pulse labelling approach
depends on assaying the biosynthetic product for extent of 13C-labelling by n.m.r.
The results lead to discussion of the late stages of the B12 biosynthetic pathway. This highlights the
need for a simple scheme of nomenclature for the various biosynthetic intermediates and a systematic
one based on the parent names precorrin and corrin is proposed.
.
.
.
.
.
.
.
.
.
.
.
.
25 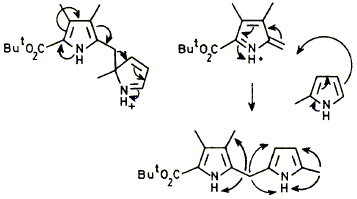
A. R. Battersby, M. G. Baker, H. A. Broadbent, C. J. R. Fookes and F. J. Leeper
"Biosynthesis of Porphyrins and Related Macrocycles, Part 29. Synthesis and Chemistry of 2,2-Disubstituted 2H-Pyrroles (Pyrrolenines)"
J. Chem. Soc., Perkin Trans. 1, 1987, 2027-2048.
Full Text
Syntheses are described of three 2H-pyrroles (pyrrolenines), (15), (28), and (64), which were designed to test the chemical feasibility of the rearrangements proposed as part of the mechanism of the enzyme cosynthetase (uroporphyrinogen Ill synthase). All three syntheses create the 2H-pyrrole ring by the Michael addition of a nitronate anion to an a,b-unsaturated ester and one introduces an additional substituent by novel alkylations of the dianion of a hydroxamic acid. Some of the intermediates in the syntheses showed unusual n.m.r. properties which reveal strong conformational preferences. The rearrangement of the 2H-pyrroles was studied under both thermal and acid-catalysed conditions. The results show that 2,2-disubstituted 2H-pyrroles only rearrange easily by [1,5]-sigmatropic shifts if they do not have further substituents on C-3 and C-4. 2-Pyrrolylmethyl-2H-pyrroles prefer to rearrange by a fragmentation-recombination mechanism.
.
.
.
.
.
.
.
.
.
.
.
.
26 
F. J. Leeper
"The Biosynthesis of Porphyrins, Chlorophylls, and Vitamin B12"
Natural Products Reports, 1987, 4, 441-469.
Full Text
Reviewing the literature published during 1985.
.
.
.
.
.
.
.
.
.
.
.
.
27 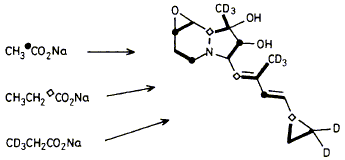
F. J. Leeper, P. Padmanabhan, G. W. Kirby and G. N. Sheldrake
"Biosynthesis of the Indolizidine Alkaloid, Cyclizidine"
J. Chem. Soc., Chem. Commun., 1987, 505-506.
Full Text
The 1H and 13C n.m.r. spectra of cyclizidine (1) have been completely assigned and incorporation experiments with 13C-labelled sodium acetate and propionate and sodium [3-2H3]propionate have revealed that the entire carbon skeleton of (1) is derived from these precursors by a polyketide-type biosynthetic pathway, with the cyclopropyl ring being derived from a single propionate unit.
.
.
.
.
.
.
.
.
.
.
.
.
28 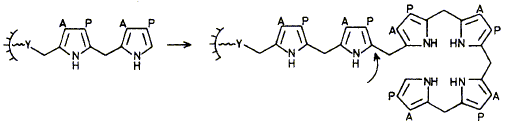
G. J. Hart, A. D. Miller, F. J. Leeper and A. R. Battersby
"Biosynthesis of the Natural Porphyrins: Proof that Hydroxymethylbilane Synthase (Porphobilinogen Deaminase) uses a Novel Binding Group in its Catalytic Action"
J. Chem. Soc., Chem. Commun., 1987, 1762-1764.
Full Text
Hydroxymethylbilane synthase builds a bilane by assembling 4 monopyrrolic units, the first of these being bound covalently to the enzyme through a group X; it is proved that X represents a unique enzymic cofactor based on a pyrromethane system.
.
.
.
.
.
.
.
.
.
.
.
.
29 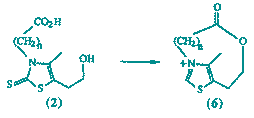
F. J. Leeper and D. H. C. Smith
"Synthesis and Structure of Bridged Thiazolium Salts"
Tetrahedron Lett.,1988, 29, 1325-1328.
Full Text
Bridged thiazolium salts (6, n = 4 and 5) have been synthesized using macrolactonisation techniques to make the corresponding thiazolinethiones followed by treatment with H2O2. For each compound the bridge exists predominantly in a single conformation which has been determined both by n.m.r. and X-ray crystallography.
.
.
.
.
.
.
.
--- Previous ten abstracts ------ Next ten abstracts ---
.