
.

.
.
 100
100
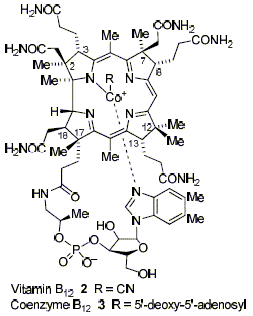
A. R. Battersby and F. J. Leeper
"Biosynthesis of B12 in the Aerobic Organism Pseudomonas denitrificans"
in Chemistry and Biochemistry of B12 (2nd Edn), R. Banerjee, Editor. 1999, J. Wiley & Sons, New York, p. 507-535.
While there are natural products now known which are bigger and therefore arguably more complex, their biosynthesis appears to be relatively straightforward in terms of the chemistry involved. For vitamin B12, on the other hand, the chemistry involved appeared at the outset to be anything but straightforward and certain to involve reactions and intermediates which were completely new to both synthetic and enzymic chemistry. The biosynthesis of B12 has therefore been described as the Everest of biosynthetic problems. In this chapter we will describe the complete pathway that has now been elucidated for the aerobic organism, Pseudomonas denitrificans. It does indeed involve much fascinating, novel and totally unexpected chemistry.
.
.
.
.
.
.
.
 101
101
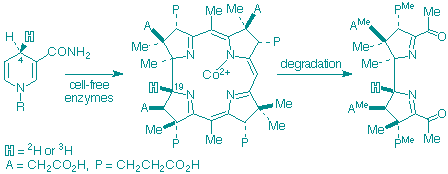
K. Ichinose, M. Kodera, F. J. Leeper and A. R. Battersby
"Biosynthesis of porphyrins and related macrocycles. Part 51. Proof that a reductive step occurs during the biosynthesis of vitamin B12 by the micro-aerophilic organism, Propionibacterium shermanii"
J. Chem. Soc., Perkin Trans. 1,1999, 879-887.
Full Text
5-Amino[4-13C]laevulinic acid has been synthesised for enzymic conversion into 13C-labelled precorrin-2. This was incubated with an enzyme system from Propionibacterium shermanii in the presence of [4-2H2]NADH and [4-2H2]NADPH to yield cobyrinic acid, shown to carry 2H at C-19 by appropriate 13C-NMR studies. The same reducing cofactors but now stereospecifically labelled at C-4 with tritium were similarly used to biosynthesise cobyrinic acid which was 3H-labelled from the 4(R)-cofactors but carried no 3H when the 4(S)-cofactors were used. Suitable degradation of the cobyrinic acid after conversion into its ester proved 3H-labelling at C-19. These results establish that the biosynthesis of vitamin B12 in the microaerophilic organism Pr. shermanii involves a reductive step in which a reductase enzyme transfers 4-HR of the cofactor to C-19 of the macrocycle.
.
.
.
.
.
.
.
 102
102
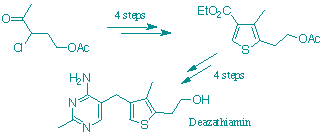
D. Hawksley, D. A. Griffin and F. J. Leeper
"Synthesis of 3-Deazathiamine."
J. Chem. Soc., Perkin Trans. 1, 2001, 144-148.
Full Text
An efficient ten-step synthesis of deazathiamin is described. The synthesis starts from commercially available a-acetyl-g-butyrolactone and proceeds via deamination of the key aminothiophene 6. The Gewald synthesis of thiophenes is shown to give a mixture of isomeric products with the unsymmetric ketone used here and so a modified procedure giving a single isomer is developed. View Crystal Structure (requires Chime)
.
.
.
.
.
.
 103
103
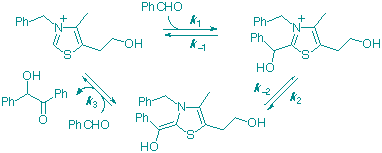
M. J. White and F. J. Leeper
"Kinetics of the Thiazolium Ion-Catalysed Benzoin Condensation."
J. Org. Chem., 2001, 66, 5124-5131.
Full Text
The formation of benzoin (Ph-CHOH-CO-Ph) from two molecules of benzaldehyde, catalyzed by 3-benzyl-5-(2-hydroxyethyl)-4-methylthiazolium bromide in methanol buffered with Et3N/Et3NH+.Cl- has been studied. Initial-rate studies at various concentrations of PhCHO (0.1-1.7 M) showed that the reaction is close to being first order in PhCHO. Following the reaction in deuteriomethanol, 1H NMR spectroscopy allowed rate constants for all three kinetically significant steps to be determined. These show that all three steps are partially rate-determining. A normal deuterium kinetic isotope effect for the overall reaction (kH/kD = ca. 3.4) is observed using PhCDO, and a large inverse solvent isotope effect (kD/kH = ca. 5.9) is observed using deuteriomethanol, consistent with the kinetic scheme presented here.
.
.
.
.
 104
104
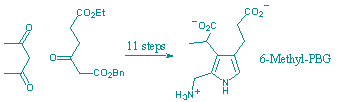
R. Ahmed and F. J. Leeper
"A new synthesis of porphobilinogen analogues, inhibitors of hydroxymethylbilane synthase."
Org. Biomolec. Chem.,, 2003, 1, 21-23.
Full Text
Two analogues of porphobilinogen, the 6-methyl and 6,11-ethano derivatives, have been made by a new synthetic route and the 6-methyl analogue has proved to be the most potent inhibitor of hydroxymethylbilane synthase yet reported (Ki = 3 mM).
.
.
.
.
.
.
.
.
 105
105
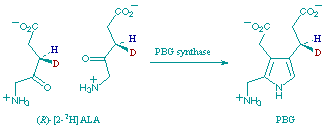
C. E. Goodwin and F. J. Leeper
"Stereochemistry and Mechanism of the Conversion of 5-Aminolaevulinic Acid into Porphobilinogen Catalysed by Porphobilinogen Synthase."
Org. Biomolec. Chem.,, 2003, 1, 1443-1446.
Full Text
(3R)- and (3S)-Deuteriated forms of 5-aminolaevulinic acid have been synthesised and the (3R)-form shows a significantly larger isotope effect when incubated with porphobilinogen synthase from bovine liver and from Bacillus subtilis; based on this and on available crystal structures, a modified mechanism for the enzymic reaction is proposed.
.
.
.
.
.
.
.
.
 106
106
S. Mann, C. Perez Melero, D. Hawksley and F. J. Leeper
"Inhibition of thiamin diphosphate dependent enzymes by 3-deazathiamin diphosphate."
Org. Biomolec. Chem.,, 2004, 2 (12), 1732-1741.
Full Text
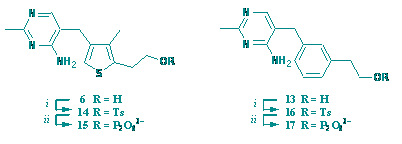
3-Deazathiamin diphosphate (deazaTPP) and a second thiamin diphosphate (TPP) analogue having a benzene ring in place of the thiazolium ring have been synthesised. These compounds are both extremely potent inhibitors of pyruvate decarboxylase from Zymomonas mobilis; binding is competitive with TPP and is essentially irreversible even though no covalent linkage is formed. DeazaTPP binds approximately seven-fold faster than TPP and at least 25,000-fold more tightly (Ki less than 14 pM). DeazaTPP is also a potent inhibitor of the E1 subunit of 2-ketoglutarate dehydrogenase from E. coli and binds more than 70-fold faster than TPP. In this case slow reversal of the inhibition could be observed and a Ki value of about 5 nM was calculated (ca. 500-fold tighter binding than TPP).
.
.
.
.
107
A. K. P. Harris, N. R. Williamson, H. Slater, A. Cox, S. Abbasi, I. Foulds, H. T. Simonsen, F. J. Leeper and G. P. C. Salmond
"The Serratia gene cluster encoding biosynthesis of the red antibiotic, prodigiosin, shows species- and strain-dependent genome context variation."
Microbiology,, 2004, 150, 3547-3560.
Full Text
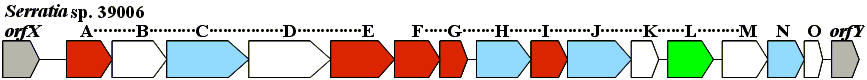
| The prodigiosin biosynthesis gene cluster (pig cluster) from two strains of Serratia (S. marcescens ATCC 274 and Serratia sp. ATCC 39006) have been cloned, sequenced, and expressed in heterologous hosts. Sequence analysis of the respective pig clusters revealed 14 ORFs, in S. marcescens ATCC 274 and 15 ORFs in Serratia sp. ATCC 39006. In each Serratia sp. predicted gene products showed similarity to polyketide synthases (PKSs), non-ribosomal peptide synthases (NRPSs) and the Red proteins of Streptomyces coelicolor A3(2). Comparisons between the two Serratia pig clusters and the red cluster from Str. coelicolor A3(2) revealed some important differences. We propose a modified scheme for the biosynthesis of prodigiosin, based on the pathway recently suggested for the synthesis of undecylprodigiosin. We demonstrate the distribution of the pig cluster within several Serratia sp. isolates and show the presence of cryptic clusters in some strains. The pig cluster of Serratia marcescens ATCC 274 is flanked by cueR and copA homologues and this configuration is demonstrated in several S. marcescens strains, whilst these genes are contiguous in strains lacking the pig cluster. |
Photograph of wild-type strains of Serratia marcescens ATCC 274, Serratia sp. ATCC 39006 and different Serratia sp. ATCC 39006 pig biosynthetic mutants grown on peptone glycerol medium. The strains shown are (from the top, working clockwise), Serratia 39006 pig biosynthetic mutant, Serratia 39006 wild-type, three Serratia 39006 pig biosynthetic mutants and S. marcescens ATCC 274 wild-type. The pigmented edges of the three pig biosynthetic mutants in the lower half of the agar plate result from where cross-feeding of biosynthetic intermediates has occurred. Photograph courtesy Dr Neil R. Williamson, Department of Biochemistry, University of Cambridge. |
.
.
.
.
.
.
.

 108
108
N. R. Williamson, H. T. Simonsen, R. A. A. Ahmed, G. Goldet, H. Slater, L. Woodley, F. J. Leeper and G. P. C. Salmond,
"Biosynthesis of the red antibiotic, prodigiosin, in Serratia: identification of a novel 2-methyl-3-n-amyl-pyrrole (MAP) assembly pathway, definition of the terminal condensing enzyme, and implications for undecylprodigiosin biosynthesis in Streptomyces."
Molec. Microbiology, 2005, 56 (4), 971-989.
Full Text
The biosynthetic pathway of the red-pigmented antibiotic, prodigiosin, produced by Serratia sp. is known to involve separate pathways for the production of the monopyrrole, 2-methyl-3-n-amyl-pyrrole (MAP) and the bipyrrole, 4-methoxy-2,2'-bipyrrole-5-carbaldehyde (MBC) which are then coupled in the final condensation step. We have previously reported the cloning, sequencing and heterologous expression of the pig cluster responsible for prodigiosin biosynthesis in two Serratia sp. In this article we report the creation of in-frame deletions or insertions in every biosynthetic gene in the cluster from Serratia sp. ATCC 39006. The biosynthetic intermediates accumulating in each mutant have been analysed by LC-MS, cross-feeding and genetic complementation studies. Based on these results we assign specific roles in the biosynthesis of MBC to the following Pig proteins: PigI, PigG, PigA, PigJ, PigH, PigM, PigF and PigN. We report a novel pathway for the biosynthesis of MAP, involving PigD, PigE and PigB. We also report a new chemical synthesis of MAP and one of its precursors, 3-acetyloctanal. Finally, we identify the condensing enzyme as PigC. We reassess the existing literature and discuss the significance of the results for the biosynthesis of undecylprodigiosin by the Red cluster in Streptomyces coelicolor A3(2).
.
.
.
.
.
.
109
F. J. Leeper, D. Hawksley, S. Mann, C. Perez Melero and M. D. H. Wood, "Studies on Thiamine Diphosphate-dependent Enzymes." Biochem. Soc. Trans., 2005, 33 (4) 772-775.
Full Text
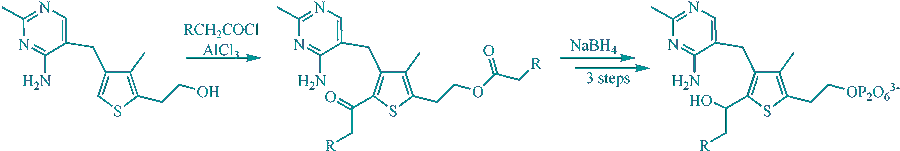
The 3-deaza analogue of thiamine diphosphate (TPP), a close mimic of the ylid intermediate, has been synthesised and is an extremely potent inhibitor of a variety of TPP-dependent enzymes, binding much more tightly than TPP itself. Results using deazaTPP complexed to the E1 subunit of pyruvate dehydrogenase have led to a novel proposal about the mechanism of this enzyme. 2-Substituted forms of deazaTPP, which mimic other intermediates in the catalytic mechanism, can also be synthesised and 2-(1-hydroxyethyl)deazaTPP is also an extremely potent inhibitor of pyruvate decarboxylase. Attachment of such 2-substituents is expected to be a way to introduce selectivity in the inhibition of the various different TPP-dependent enzymes.
.
.
.
--- Next ten abstracts ----- Previous ten abstracts ---
.
.