
.
110
 N. R. Williamson, H. T. Simonsen, A. K. P. Harris, F. J. Leeper and George P. C. Salmond, "Disruption of the copper efflux pump (CopA) of Serratia marcescens ATCC 274 pleiotropically affects copper sensitivity and production of the tripyrrole secondary metabolite, prodigiosin." J. Indust. Microbiol. Biotechnol., 2006, 33 (2), 151-158.
Full Text
N. R. Williamson, H. T. Simonsen, A. K. P. Harris, F. J. Leeper and George P. C. Salmond, "Disruption of the copper efflux pump (CopA) of Serratia marcescens ATCC 274 pleiotropically affects copper sensitivity and production of the tripyrrole secondary metabolite, prodigiosin." J. Indust. Microbiol. Biotechnol., 2006, 33 (2), 151-158.
Full Text
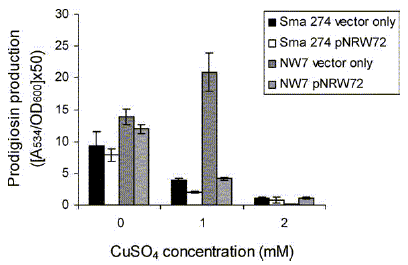
The prodigiosin biosynthetic gene cluster (pig cluster) of Serratia marcescens ATCC 274
(Sma 274) is flanked by cueR/copA homologues. Inactivation of the copA homologue
resulted in an increased sensitivity to copper, confirming that CopA is involved in copper
homeostasis in Sma 274. The affect of copper on the biosynthesis of prodigiosin in Sma
274 and the copA mutant strain was investigated. Increased levels of copper were found
to reduce prodigiosin production in the wild type Sma 274, but increase production in the
copA mutant strain. The physiological implications for CopA mediated prodigiosin
production are discussed. We also demonstrate that the gene products of pigB-pigE of
Sma 274 are sufficient for the biosynthesis of 2-methyl-3-n-amyl-pyrrole (MAP) and
condensation with 4-methoxy-2,2'-bipyrrole-5-carboxyaldehyde (MBC) to form
prodigiosin, as we have shown for Serratia sp. ATCC 39006.
.
.
.
.
111
Neil R. Williamson, Peter C. Fineran, Finian J. Leeper and George P. C. Salmond, "The Regulation and Biosynthesis of Bacterial Prodiginines." Nature Rev. Microbiol., 2006, 4, 887-899.
Full Text
The red-pigmented prodiginines are bioactive secondary metabolites produced by both Gram-negative and Gram-positive bacteria. Recently, these tripyrrole molecules have received renewed attention owing to reported immunosuppressive and anticancer properties. The enzymes involved in the biosynthetic pathways for the production of two of these molecules, prodigiosin and undecylprodigiosin, are now known. However, the biochemistry of some of the reactions is still poorly understood. The physiology and regulation of prodiginine production in Serratia and Streptomyces are now well understood, although the biological role of these pigments in the producer organisms remains unclear. However, research into the biology of pigment production will stimulate interest in the bioengineering of strains to synthesize useful prodiginine derivatives.
|

Summary of the genetic regulation of prodigiosin production in Serratia species. Activation is represented by triangular arrow-heads, repression is shown by flat arrow-heads.
|
.
.
.
.
 112
112
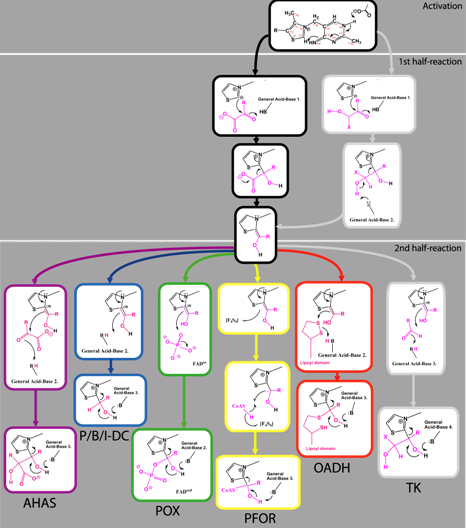
René Frank, Finian Leeper and Ben F. Luisi, "Structure, mechanism and catalytic duality of thiamine-dependent enzymes." Cell. Molec. Life Sci., 2006, 64, 892-905.
Full Text
Thiamine is an essential cofactor that is required for processes of general metabolism amongst all organisms, and it is likely to have played a role in the earliest stages of the evolution of life. Here, we review from a structural aspect the enzymatic mechanisms that involve the cofactor and the origins or their specificity. We explore asymmetry within homo-dimeric thiamine diphosphate (ThDP)-dependent enzyme structures and discuss how this may be correlated with the kinetic properties of half-of-the-sites-reactivity, and negative cooperativity. It is likely these structural and kinetic hallmarks may arise through reciprocal coupling of active sites. This mode of communication between distant active sites is not unique to ThDP-dependent enzymes, but is widespread in other classes of oligomeric enzyme. Thus it appears likely that to be a general phenomenon reflecting a powerful mechanism of accelerating the rate of a chemical pathway. Finally, we speculate on the early evolutionary history of the cofactor and its ancient association with protein and RNA.
.
.
.
.
 113
113
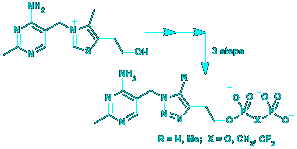
Karl M. Erixon, Chester L. Dabalos and Finian Leeper, "Inhibition of pyruvate decarboxylase from Z. mobilis by novel analogues of thiamine pyrophosphate: investigating pyrophosphate mimics." Chem. Comm., 2007, 960-962.
Full Text
Replacement of the thiazolium ring of thiamine pyrophosphate with a triazole gives extremely potent inhibitors of pyruvate decarboxylase from Z. mobilis, with KI values down to 20 pM; this system was used to explore pyrophosphate mimics and several effective analogues were discovered.
.
.
.
.
 114
114
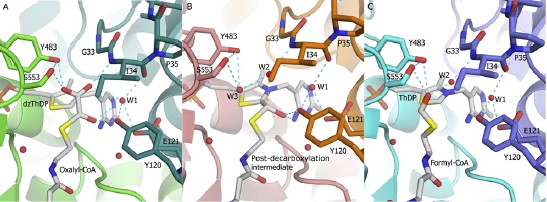
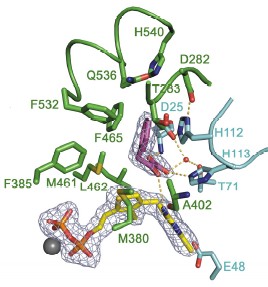
Catrine L. Berthold, Cory G. Toyota, Patricia Moussatche, Martin D. Wood, Finian J. Leeper, Nigel G. J. Richards, Ylva Lindqvist, "Crystallographic snapshots of oxalyl-CoA decarboxylase give new insights into catalysis by non-oxidative ThDP-dependent decarboxylases." Structure, 2007, 15 (7), 853-861.
Full Text
Despite more than five decades of extensive studies of thiamin diphosphate (ThDP) enzymes,
there remain many uncertainties as to how these enzymes achieve their rate enhancements.
Here, we present a clear picture of catalysis for the simple nonoxidative decarboxylase,
oxalyl-coenzyme A (CoA) decarboxylase, based on crystallographic snapshots along the
catalytic cycle and kinetic data on active site mutants. First, we provide crystallographic
evidence that, upon binding of oxalyl-CoA, the C-terminal 13 residues fold over the substrate,
aligning the substrate a-carbon for attack by the ThDP-C2 atom. The second structure presented
shows a covalent reaction intermediate after decarboxylation, interpreted as being
nonplanar. Finally, the structure of a product complex is presented. In accordance with
mutagenesis data, no side chains of the enzyme are implied to directly participate in proton
transfer except the glutamic acid (Glu-56), which promotes formation of the 1',4'-iminopyrimidine
tautomer of ThDP needed for activation.
.
.
.
.
 115
115
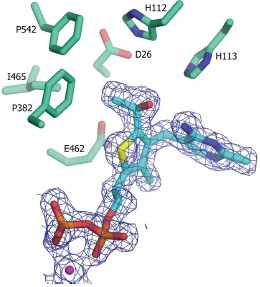
Wim Versées, Stijn Spaepen, Martin D. H. Wood, Finian J. Leeper, Jos Vanderleyden and Jan Steyaert, "Molecular mechanism of allosteric substrate activation in a thiamine diphosphate-dependent decarboxylase." J. Biol. Chem., 2008, in press.
Full Text.
Thiamine diphosphate-dependent enzymes
are involved in a wide variety of metabolic
pathways. The molecular mechanism behind
active site communication and substrate
activation, observed in some of these
enzymes, has since long been an area of
debate. Here, we report the crystal structures
of a phenylpyruvate decarboxylase in
complex with its substrates and a covalent
reaction intermediate analogue. These
structures reveal the regulatory site and
unveil the mechanism of allosteric substrate
activation. This signal transduction relies on
quaternary structure reorganizations,
domain rotations and a pathway of local
conformational changes that are relayed from
the regulatory site to the active site. The
current findings thus uncover the molecular
mechanism by which the binding of a
substrate in the regulatory site is linked to the
mounting of the catalytic machinery in the
active site in this ThDP-dependent enzyme.
.
.
.
.
 116
116
Finian J. Leeper and Alison G. Smith, "Editorial: Vitamins and cofactors—chemistry, biochemistry and biology." Nat. Prod. Rep., 2007, 24 (5), 923–926.
Full Text.
.
.
.
.
 117
117
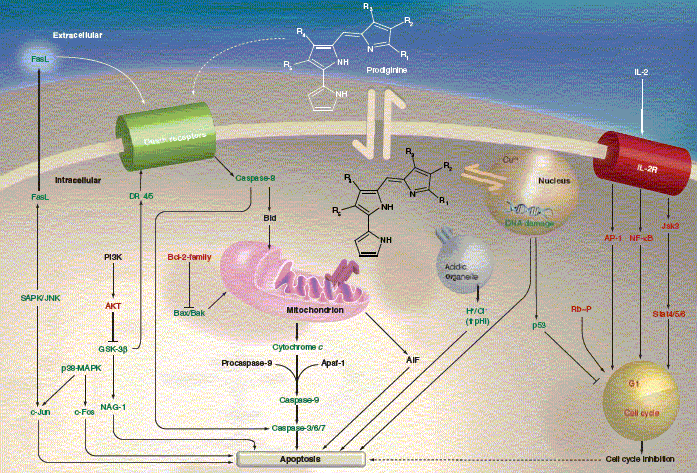
Neil R. Williamson, Peter C. Fineran, T. Gristwood, Suresh R. Chawrai, Finian J. Leeper and George P. C. Salmond, "Anticancer and Immunosuppressive Properties of Bacterial Prodiginines." Future Microbiology, 2007, 2 (6), 605-618.
Full Text.
Bacterial prodiginines are a family of red-pigmented, tripyrrolic compounds, which show
numerous biological activities including antibacterial, antifungal, antiprotozoal,
antimalarial, immunosuppressive and anticancer properties. Recently, significant progress
has been made on understanding the biosynthesis and regulation of bacterial prodiginines.
An understanding of the biosynthesis of prodiginines will allow engineering of bacterial
strains capable of synthesising novel prodiginines through rational design and
mutasynthesis experiments. Bacterial prodiginines and synthetic derivatives are effective
proapoptotoic agents with multiple cellular targets, active against numerous cancer cell
lines including multidrug resistant cells with little or no toxicity towards normal cell lines.
A synthetic derivative, GX15-070 (Obatoclax), developed through structure activity
relationship (SAR) studies of pyrrole ring A of butyl-meta-cycloheptylprodiginine, is in
multiple phase I and phase II clinical trials in both single and dual agent studies to treat
different types of cancer. Therefore prodiginines have real therapeutic potential in the clinic.
.
.
.
.
 118
118
Catrine L. Berthold, Dörte Gocke, Martin D. Wood, Finian J. Leeper, Martina Pohl and Gunter Schneider, "Structure of the branched-chain keto acid decarboxylase (KdcA) from Lactococcus lactis provides insights into the structural basis for the chemoselective and enantioselective carboligation reaction." Acta Cryst., D 2007, 63 (12), 1217–1224.
Full Text.
The thiamin diphosphate (ThDP) dependent branched-chain
keto acid decarboxylase (KdcA) from Lactococcus lactis
catalyzes the decarboxylation of 3-methyl-2-oxobutanoic acid
to 3-methylpropanal (isobutyraldehyde) and CO2. The
enzyme is also able to catalyze carboligation reactions with
an exceptionally broad substrate range, a feature that makes
KdcA a potentially valuable biocatalyst for C—C bond
formation, in particular for the enzymatic synthesis of
diversely substituted 2-hydroxyketones with high enantioselectivity.
The crystal structures of recombinant holo-KdcA
and of a complex with an inhibitory ThDP analogue
mimicking a reaction intermediate have been determined to
resolutions of 1.6 and 1.8 Å, respectively. KdcA shows the
fold and cofactor–protein interactions typical of thiamin-dependent
enzymes. In contrast to the tetrameric assembly
displayed by most other ThDP-dependent decarboxylases of
known structure, KdcA is a homodimer. The crystal structures
provide insights into the structural basis of substrate
selectivity and stereoselectivity of the enzyme and thus are
suitable as a framework for the redesign of the substrate
profile in carboligation reactions.
.
.
.
.
 119
119
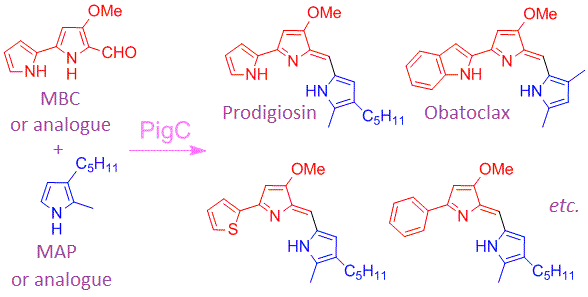
Suresh R. Chawrai, Neil R. Williamson, George P. C. Salmond and Finian J. Leeper,
"Chemoenzymatic synthesis of prodigiosin analogues – exploring the substrate specificity of PigC."
Chem. Commun., 2008, 1862–1864. Full Text.
Analogues of prodigiosin, a tripyrrolic pigment produced by Serratia species with potent immunosuppressive and anticancer activities, have been produced by feeding synthetic analogues of the normal precursor MBC to mutants of Serratia sp. ATCC 39006 or to engineered strains of Escherichia coli; in this way it has been shown that the prodigiosin synthesising enzyme, Pig C, has a relaxed substrate-specificity.
--- Next ten abstracts --- . . . --- Previous ten abstracts ---
.
.
 N. R. Williamson, H. T. Simonsen, A. K. P. Harris, F. J. Leeper and George P. C. Salmond, "Disruption of the copper efflux pump (CopA) of Serratia marcescens ATCC 274 pleiotropically affects copper sensitivity and production of the tripyrrole secondary metabolite, prodigiosin." J. Indust. Microbiol. Biotechnol., 2006, 33 (2), 151-158.
Full Text
N. R. Williamson, H. T. Simonsen, A. K. P. Harris, F. J. Leeper and George P. C. Salmond, "Disruption of the copper efflux pump (CopA) of Serratia marcescens ATCC 274 pleiotropically affects copper sensitivity and production of the tripyrrole secondary metabolite, prodigiosin." J. Indust. Microbiol. Biotechnol., 2006, 33 (2), 151-158.
Full Text


 112
112 113
113 114
114 115
115 116
116 117
117 118
118 119
119