
.

.
.
 70
70
F. Blanche, B. Cameron, J. Crouzet, L. Debussche, D. Thibaut, M. Vuilhorgne, F. J. Leeper and A. R. Battersby
"Vitamin B12: How the Problem of its Biosynthesis was Solved"
Angew. Chem., Int. Ed. Engl.,1995, 34, 383-411.
Full Text
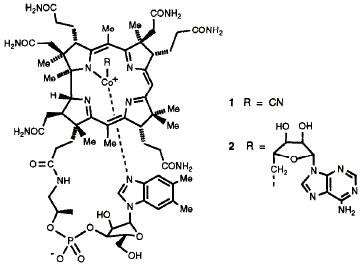
Vitamin B12 is an essential vitamin for human health and lack of it leads to pernicious anaemia. This biological activity has attracted intense interest, and continues to do so; in addition, the complex architecture of the B12 molecule has fascinated chemists and biochemists since its discovery as the first natural organo-cobalt complex and the establishment of its structure by X-ray analysis. The organic ligand surrounding the cobalt displays many stereogenic centres around its periphery carrying reactive functional groups. This complexity led B12 to be rightly regarded as an extreme challenge to the synthetic chemist. Yet micro-organisms achieve this synthesis in vivo with complete control of regio- and stereochemistry. How do they do it? This Review tells the full remarkable story. Success in unravelling this biosynthetic puzzle came from a collaborative effort by biologists and chemists using the full range of approaches available from their disciplines. These extended from genetics at one end of the spectrum to synthesis and NMR at the other. This work can act as a guide for future research on the biosynthesis of yet more complex natural substances.
.
.
.
.
.
.
.
.
.
.
71 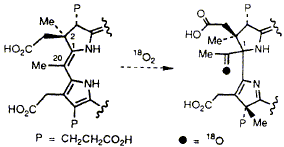 A. I. D. Alanine, Y. Li, N. P. J. Stamford, F. J. Leeper, F. Blanche, L. Debussche and A. R. Battersby
A. I. D. Alanine, Y. Li, N. P. J. Stamford, F. J. Leeper, F. Blanche, L. Debussche and A. R. Battersby
"Biosynthesis of Vitamin B12: Studies of the Oxidative and Lactone-forming Steps by 18O-Labelling"
J. Chem. Soc., Chem. Commun.,1994, 1649-1650.
Full Text
Experiments based on the use of 18O2 and electrospray mass spectrometry show that during the conversion of precorrin-3A into precorrin-3B or precorrin-4 just one atom of oxygen is incorporated.
.
.
.
.
.
.
.
.
.
.
72 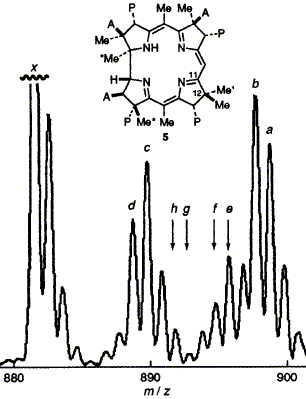 Y. Li, A. I. D. Alanine, S. Balachandran, R. A. Vishwakarma, F. J. Leeper and A. R. Battersby
Y. Li, A. I. D. Alanine, S. Balachandran, R. A. Vishwakarma, F. J. Leeper and A. R. Battersby
"Biosynthesis of Vitamin B12: Mechanistic Studies on the Transfer of a Methyl Group from C-11 to C-12 and Incorporation of 18O"
J. Chem. Soc., Chem. Commun.,1994, 2507-2508.
Full Text
The transfer of a methyl group from C-11 to C-12 in vitamin B12 biosynthesis is shown, by a crossover experiment involving [1,11,17- (CD3)3]-labelled precursors, to occur in an intramolecular fashion; it is found that in Pseudomonas denitrificans, unlike Propionibacterium shermanii, no exchange of the ring A acetate oxygen atoms occurs during formation of the corrin ring.
.
.
.
.
.
.
.
.
.
73 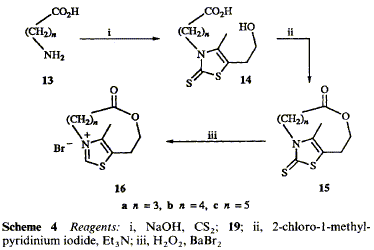 F. J. Leeper and D. H. C. Smith
F. J. Leeper and D. H. C. Smith
"Bridged Thiazolium Salts as Models for Thiamin: Synthesis and Reactions"
J. Chem. Soc., Perkin Trans. 1,1995, 861-873.
Full Text
Bridged thiazolium salts 16b and c have been synthesized in a short procedure starting with w-amino acids. Condensation with carbon disulphide and a-chloroketone 19 provided thiazoline-2-thiones 14b and c and the bridge was then formed by standard macrolactonization procedures. Oxidation of the thiones with hydrogen peroxide then gave the thiazolium salts. The same cyclization procedures failed to yield the shorter bridged compound 16a but gave the cyclic dimer and trimer. Starting with protected lysine derivatives 31b and c, thiazoline-2-thiones 36b and c were obtained. In both cases the cyclization reaction yielded two separable atropisomers depending on which side of the ring the bridge was formed. The catalytic reactions of thiazolium salts 16b and c were compared with unbridged analogues and it was found that, whereas the longer bridged one behaved normally, the shorter bridged salt was unable to catalyse the benzoin condensation. A novel 2-benzoyl-2,3-dihydrothiazole 44 was isolated from this reaction mixture.
.
.
.
.
.
.
.
.
.
.
74 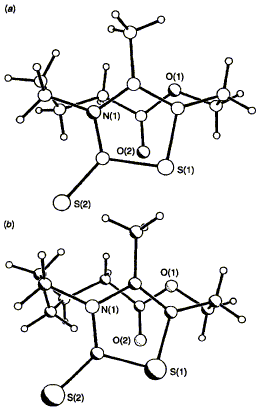 F. J. Leeper, D. H. C. Smith, M. J. Doyle and P. R. Raithby
F. J. Leeper, D. H. C. Smith, M. J. Doyle and P. R. Raithby
"Bridged Thiazolium Salts as Models for Thiamin: NMR, Crystallographic and Molecular Mechanics Studies"
J. Chem. Soc., Perkin Trans. 2,1995, 777-784.
Full Text
NMR data for the bridged thiazolium salts 1b and c and thiazolinethiones 2b and c are described which allows their conformations in solution to be deduced. These are in full agreement with the X-ray crystal structures for 2b and c. The structures and conformations of the two atropisomers of the substituted thiazolinethiones 6 and 7 have also been deduced. Molecular mechanics calculations agree with the deduced conformations and have been used to predict the strain involved in the bridged thiazolium salts and the degree to which this strain is relieved in some of their reactions.
.
.
.
.
.
.
.
.
.
.
75 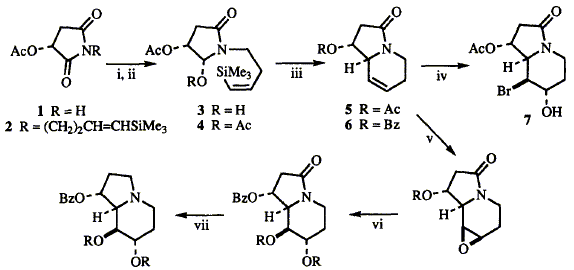 F. J. Leeper and S. Howard
F. J. Leeper and S. Howard
"Stereocontrolled Syntheses of Polyhydroxy Indolizidines, Including 8a-Epi, 6,8a-Diepi and 1,6-Diepi-castanospermine, Starting from Malic Acid"
Tetrahedron Lett.,1995, 36, 2335-2338.
Full Text
Stereocontrolled total syntheses of one trihydroxy indolizidine and three tetrahydroxy indolizidines - all diastereoisomers of castanospermine - are described which use malic acid as the only chiral starting material.
.
.
.
.
.
.
.
.
.
.
76 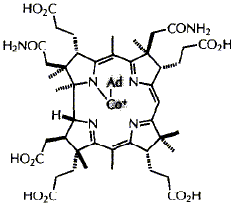 F. Kiuchi, F. J. Leeper and A. R. Battersby
F. Kiuchi, F. J. Leeper and A. R. Battersby
"Biosynthesis of Porphyrins and Related Macrocycles, Part 43. Isolation and Characterisation of Intermediates of Coenzyme B12 Biosynthesis, a Cobyrinic Acid Triamide, the a,c-diamide and their Co-(5'-Deoxy-5'-adenosyl) derivatives, from Propionibacterium shermanii"
Chemistry & Biology,1995, 2, 527-532.
Vitamin B12 is biosynthesised by many different organisms e.g. Pseudomonas
denitrificans (aerobic) and Propionibacterium shermanii (essentially
anaerobic, "microaerophilic"). The biosynthetic pathways in these two organisms
show strong similarities but also some differences. Here we focus on the stages
beyond the formation of the corrin macrocycle, about which there had earlier
been conflicting reports.
A single cobyrinic acid diamide and a single triamide have been isolated from
Pr. shermanii and the diamide was shown to be the a,c-isomer. The triamide
is not the a,c,g-isomer but it is indistinguishable from the single triamide
isolated by other workers from Ps. denitrificans. Also Co-(5'-deoxy-5'-adenosyl)cobyrinic
acid a,c-diamide has been isolated and fully characterised and the deoxyadenosyl
derivative of the foregoing triamide has been shown to be present in the cells.
Our results support a unique pathway in Pr. shermanii going forward from
cobyrinic acid towards vitamin B12, at least as far as the adenosylated
triamide intermediate. No evidence was found for multiple alternative pathways.
.
.
.
.
.
.
.
.
.
.
77 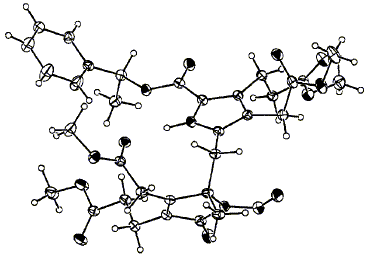 A. C. Spivey, A. Capretta, C. S. Frampton, F. J. Leeper and A. R. Battersby
A. C. Spivey, A. Capretta, C. S. Frampton, F. J. Leeper and A. R. Battersby
"Stereochemical Studies on the Proposed Spiro Intermediate for the Biosynthesis of the Natural Porphyrins: Determination by a Novel X-Ray Method of the Absolute Configuration of the Spirolactam which Inhibits Cosynthetase"
J. Chem. Soc., Chem. Commun.,1995, 1789-1790.
Full Text
A novel X-ray analysis, combined with correlations by circular dichroism, has been used to establish the R-configuration at the stereocentre in that enantiomer of the spirolactam which strongly inhibits uroporphyrinogen III synthase.
.
.
.
.
.
.
.
.
.
.
78 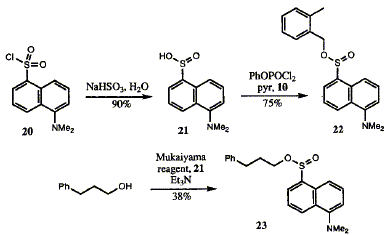 C. Lawrence, I. M. Bell, C. Abell and F. J. Leeper
C. Lawrence, I. M. Bell, C. Abell and F. J. Leeper
"Approaches to Antibody-catalysed Cationic Cyclisations: Chemical Studies of Leaving Groups and Cyclisation Modes"
Isr. J. Chem.,1996, 36, 161-169.
The solvolysis of a range of esters of 2-methylbenzyl alcohol has been studied. It was found that sulphonate esters are solvolysed very rapidly in aqueous solution (t1/2 ca. 35 s) whereas sulphinates react slowly (t1/2 ca. 35 h) and a phosphonate diester is stable. The fluorescent 5-dimethylaminonaphthalene-1-sulphinate (dansinate) esters held the most promise as substrates for antibody-catalysed cationic cyclisations. It was shown by 18O-labelling studies that ester is hydrolysed predominantly (but not exclusively) by SN1 reaction at the benzylic position. A number of conditions were tried to effect cationic cyclisations of an unsaturated benzylic alcohol, whose ester is intended as the substrate for the antibody-catalysed reaction. The products obtained were the result of attack of the non-benzylic alcohol on either the benzylic cation, to give 31 and 32, or on the protonated alkene, to give 29. No cyclisation of the alkene onto the benzylic cation, to give 4, was observed, suggesting that this is a disfavoured reaction in solution.
.
.
.
.
.
.
.
.
.
.
79 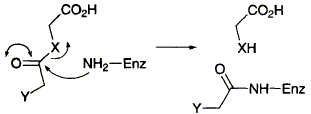 D. Appleton and F. J. Leeper
D. Appleton and F. J. Leeper
"Mechanism-based Inhibition of 5-Aminolaevulinic Acid Dehydratase from Bacillus subtilis by the 3-Thia Analogue of the Substrate"
Chem. Commun.,1996, 303-304.
Full Text
The interaction of various substrate analogues with 5-aminolaevulinic acid dehydratase (porphobilinogen synthase) from Bacillus subtilis has been studied kinetically and by electrospray mass spectrometry; 5-chlorolaevulinic acid has been shown to be a non-specific alkylating agent but 5-amino-3-thialaevulinic acid is a potent mechanism-based inactivator.
.
.
--- Next ten abstracts ----- Previous ten abstracts ---
.
.