

.
60 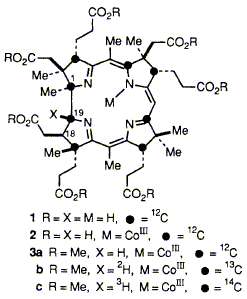 K. Ichinose, M. Kodera, F. J. Leeper and A. R. Battersby
K. Ichinose, M. Kodera, F. J. Leeper and A. R. Battersby
"Proof that the Biosynthesis of Vitamin B12 Involves a Reduction Step in an Anaerobic as well as an Aerobic Organism"
J. Chem. Soc., Chem. Commun.,1993, 515-517.
Full Text
Labelling experiments with 2H and 3H prove that as cobyrinic acid is biosynthesised by the anaerobic bacterium Propionibacterium shermanii, its H-19 is derived from HR (and not HS) at C-4 of a reduced nicotinamide cofactor, so demonstrating that the biosynthetic pathway involves a reduction step.
.
.
.
.
.
.
.
.
.
61 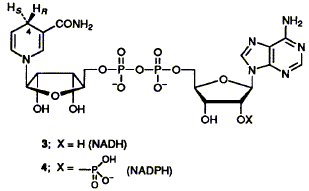 K. Ichinose, F. J. Leeper and A. R. Battersby
K. Ichinose, F. J. Leeper and A. R. Battersby
"Preparation of [4R-3H]NADH, [4R-3H]NADPH and the Corresponding 4S-Isomers All with Substantial Specific Activities"
J. Chem. Soc., Perkin Trans. 1, 1993, 1213-1216.
Full Text
Methods are developed for the enzymic preparation of [4R-3H]NADH, [4R-3H]NADPH and the corresponding 4S-isomers, all stereospecifically labelled and of specific activities amply high enough for sensitive labelling experiments. Sodium borotritiide provides the tritium for all four cofactors.
.
.
.
.
.
.
.
62 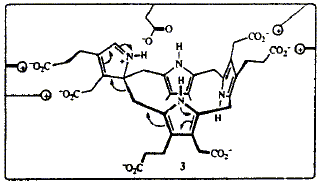 F. J. Leeper
F. J. Leeper
"Evidence for a Spirocyclic Intermediate in the Formation of Urogen III by Cosynthetase"
in The Biosynthesis of the Tetrapyrrole Pigments, 1994, J. Wiley & Sons, Chichester, p. 111-123.
Full Text
In the course of the cyclization of the linear tetrapyrrole hydroxymethylbilane to uroporphyrinogen III, catalysed by uroporphyrinogen III synthase (cosynthase), ring D of the bilane becomes inverted. Many different mechanisms have been proposed for this transformation but the most economical is one involving a spirocyclic pyrrolenine. Synthesis of a spirolactam, and other compounds closely related to the spirocyclic pyrrolenine, has shown that such compounds are not impossibly strained. The spirolactam is a powerful inhibitor of the enzyme, which suggests it does resemble an intermediate in the enzymic process. In the synthetic procedure to make an ester of the spirolactam the two products obtained were initially thought to be conformational isomers. However, molecular mechanics calculations on a model of the spirolactam predicted that several low energy conformations should exist and that the energy barriers for their interconversion are all lower than 32 kJ/mol. Reinvestigation revealed that one of the two products is in fact a macrocyclic dimer with a 28-membered ring. On the basis of the predicted preferred conformations of the spirolactam and of uroporphyrinogen III, a detailed three-dimensional mechanism is proposed, along with a rationalization of how the rearrangement of ring D may be directed by the enzyme.
.
.
.
.
.
.
.
.
.
63 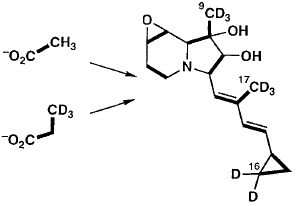 F. J. Leeper, S. E. Shaw and P. Satish
F. J. Leeper, S. E. Shaw and P. Satish
"The Biosynthesis of Cyclizidine: Incorporation of Singly and Doubly Labelled Precursors"
Can. J. Chem., 1994, 72, 131-141.
Full Text
Incorporation of isotopically labelled forms of CH3CO2Na and CH3CH2CO2Na into the indolizidine alkaloid cyclizidine, produced by Streptomyces species NCIB 11649, shows that the oxygen attached to C-2 is derived intact from acetate and that the cyclopropyl ring is derived from a single intact propionate unit. However, the level and stereochemistry of the incorporation of deuteriated sodium propionate indicates that it undergoes unexpected modification during incorporation into the cyclopropyl ring.
.
.
.
.
.
.
.
.
.
64 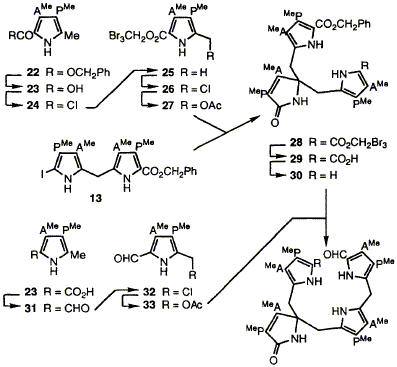 W. M. Stark, C. J. Hawker, G. J. Hart, A. Philippides, P. M. Petersen, J. D. Lewis, F. J. Leeper and A. R. Battersby
W. M. Stark, C. J. Hawker, G. J. Hart, A. Philippides, P. M. Petersen, J. D. Lewis, F. J. Leeper and A. R. Battersby
"Biosynthesis of Porphyrins and Related Macrocycles, Part 40. Synthesis of a Spiro-Lactam Related to the Proposed Spiro-Intermediate for Porphyrin Biosynthesis: Inhibition of Cosynthetase"
J. Chem. Soc., Perkin Trans. 1,1993, 2875-2892.
Full Text
Routes are developed for synthesis of the tripyrrolic macrocyclic spiro-lactam 39. A minor product from the synthesis, thought earlier to be an atropisomer, has been shown by molecular mechanics calculations and re-investigation to be a dimer. The octa-acid derived from 39 closely resembles the spiro-pyrrolenine proposed as a biosynthetic intermediate for uroporphyrinogen III. This octa-acid acts as a strong inhibitor of cosynthetase (uroporphyrinogen III synthase) whilst other similar systems which lack some of its functionality do not. These results strongly support the view that the spiro system is indeed the biosynthetic intermediate for formation of uroporphyrinogen III from hydroxymethylbilane.
.
.
.
.
.
.
.
.
.
65 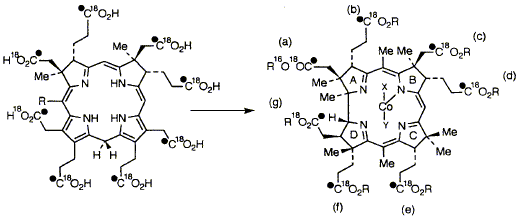 R. A. Vishwakarma, S. Balachandran, A. I. D. Alanine, N. P. J. Stamford, F. Kiuchi, F. J. Leeper and A. R. Battersby
R. A. Vishwakarma, S. Balachandran, A. I. D. Alanine, N. P. J. Stamford, F. Kiuchi, F. J. Leeper and A. R. Battersby
"Biosynthesis of Porphyrins and Related Macrocycles. Part 41. Fate of Oxygen Atoms as Precorrin-2 Carrying Eight Labelled Carboxyl Groups (13C18O2H) is Enzymatically Converted into Cobyrinic Acid"
J. Chem. Soc., Perkin Trans. 1,1993, 2893-2899.
Full Text
5-Amino[1,4-13C2]laevulinic acid and 5-amino[1-13C]laevulinic acid are synthesised and all three 16O atoms of the latter are exchanged for 18O. The 13C,18O-labelled material is then converted in vitro into precorrin-2 by the combined action of four genetically overproduced enzymes. The product is isolated in its aromatised form, sirohydrochlorin and 13C-NMR shows that all 8 carboxyl groups retain both oxygen atoms throughout the biosynthesis. A cell-free enzyme preparation from Propionibacterium shermanii converts the 13C,18O-labelled sirohydrochlorin via precorrin-2 into cobyrinic acid, a late precursor of vitamin B12. 13C-NMR proves that 6 carboxyl groups of cobyrinic acid (b-g, inclusive) retain both oxygen atoms whereas the a-carboxyl group undergoes specific loss of one labelled oxygen atom.
.
.
.
.
.
.
.
.
.
66 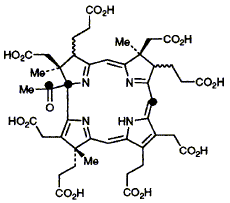 A. I. D. Alanine, K. Ichinose, D. Thibaut, L. Debussche, N. P. J. Stamford, F. J. Leeper, F. Blanche and A. R. Battersby
A. I. D. Alanine, K. Ichinose, D. Thibaut, L. Debussche, N. P. J. Stamford, F. J. Leeper, F. Blanche and A. R. Battersby
"Biosynthesis of Vitamin B12: Use of Specific 13C-Labelling for Structural Studies on Factor IV"
J. Chem. Soc., Chem. Commun.,1994, 193-196.
Full Text
[1,10,20-13C3]Uro'gen III is unambiguously synthesised for enzymic conversion into precorrin-4 isolated after aerial oxidation as two epimers of Factor IV, the 13C NMR spectra of which rigorously confirm the presence of a C-1 acetyl group in precorrin-4; attachment of the fourth methyl group at C-17 of precorrin-4 is also confirmed by related 13C-labelling experiments.
.
.
.
.
.
.
.
.
.
67 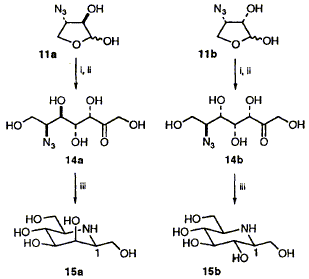 K. E. Holt, F. J. Leeper and S. Handa
K. E. Holt, F. J. Leeper and S. Handa
"Synthesis of beta-1-Homonojirimycin and beta-1-Homomannojirimycin using the Enzyme Aldolase"
J. Chem. Soc., Perkin Trans.1,1994, 231-234.
Full Text
The four stereoisomers of the four-carbon azido sugar 11 have been stereoselectively synthesised by a route involving Sharpless epoxidation and all are found to be substrates for rabbit muscle fructose 1,6-bisphosphate aldolase, giving (after treatment with phosphatase) 6-azido-6-deoxyheptuloses 14; hydrogenation of 14a and 14b gave beta-1-homomannojirimycin 15a and beta-1-homonojirimycin 15b with high selectivity.
.
.
.
.
.
.
.
.
.
68 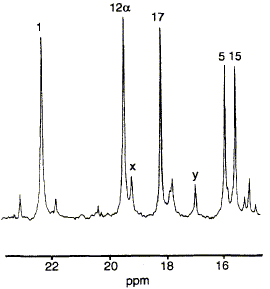 S. Balachandran, R. A. Vishwakarma, S. M. Monaghan, A. Prelle, N. P. J. Stamford, F. J. Leeper and A. R. Battersby
S. Balachandran, R. A. Vishwakarma, S. M. Monaghan, A. Prelle, N. P. J. Stamford, F. J. Leeper and A. R. Battersby
"Biosynthesis of Porphyrins and Related Macrocycles, Part 42. Pulse Labelling Experiments Concerning the Timing of Cobalt Insertion During Vitamin B12 Biosynthesis"
J. Chem. Soc., Perkin Trans. 1,1994, 487-491.
Full Text
Pulse labelling experiments using 60Co2+ and [methyl-13C,14C]-S-adenosyl-L-methionine have established that in the anaerobic organism Propionibacterium shermanii cobalt is inserted into the macrocycle after the second and before the fourth C-methylation step.
.
.
.
.
.
.
.
.
.
69 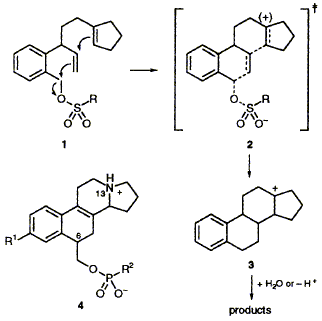 I. M. Bell, C. Abell and F. J. Leeper
I. M. Bell, C. Abell and F. J. Leeper
"Design and Synthesis of Transition State Analogues for a Cationic Cyclisation"
J. Chem. Soc., Perkin Trans.1,1994, 1997-2006.
Full Text
Transition-state analogues based upon the 6-(hydroxymethyl)-13- azagona-1,3,5(10),8-tetraene structure have been designed and synthesized as part of a programme to elicit antibodies capable of catalysing cationic cyclisations. Methodology for conjugating such analogues to proteins has also been developed.
.
.
--- Next ten abstracts ----- Previous ten abstracts ---
.
.