

.
50 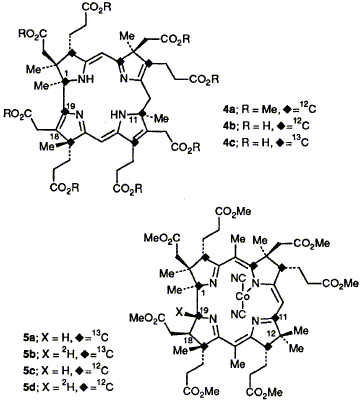 G. W. Weaver, F. J. Leeper, A. R. Battersby, F. Blanche, D. Thibaut and L. Debussche
G. W. Weaver, F. J. Leeper, A. R. Battersby, F. Blanche, D. Thibaut and L. Debussche
"Biosynthesis of Vitamin B12: the Site of Reduction of Precorrin-6x"
J. Chem. Soc., Chem. Commun.,1991, 976-979.
Full Text
It is proved by deuterium labelling and NMR spectroscopy that a hydride equivalent from NADPH is transferred to C-19 of precorrin-6x as this intermediate is converted enzymically into hydrogenobyrinic acid.
.
.
.
.
.
.
.
.
.
51 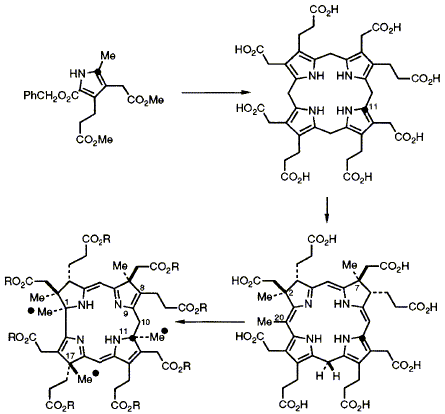 F. Blanche, M. Kodera, M. Couder, F. J. Leeper, D. Thibaut and A. R. Battersby
F. Blanche, M. Kodera, M. Couder, F. J. Leeper, D. Thibaut and A. R. Battersby
"Biosynthesis of Vitamin B12: Use of a Single 13C Label in the Macrocycle to Confirm C-11 Methylation in Precorrin-6x"
J. Chem. Soc., Chem. Commun.,1992, 138-139.
Full Text
[11-13C]Uro'gen III is unambiguously synthesised for enzymic conversion into precorrin-6x which as its octamethyl ester gives a 13C NMR spectrum confirming the presence of a C-11 methyl group.
.
.
.
.
.
.
.
.
.
52 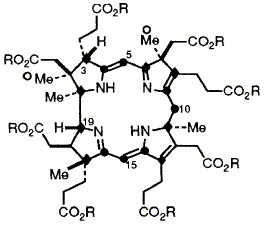 D. Thibaut, F. Kiuchi, L. Debussche, F. J. Leeper, F. Blanche and A. R. Battersby
D. Thibaut, F. Kiuchi, L. Debussche, F. J. Leeper, F. Blanche and A. R. Battersby
"Biosynthesis of Vitamin B12: Structure of the Ester of a New Biosynthetic Intermediate, Precorrin-6y"
J. Chem. Soc., Chem. Commun.,1992, 139-141.
Full Text
13C Labelling and NMR experiments establish the structure of precorrin-6y octamethyl ester, the corresponding octa-acid being a further new intermediate on the biosynthetic pathway to hydrogenobyrinic acid
.
.
.
.
.
.
.
.
.
53 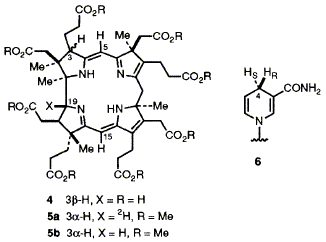 F. Kiuchi, D. Thibaut, L. Debussche, F. J. Leeper, F. Blanche and A. R. Battersby
F. Kiuchi, D. Thibaut, L. Debussche, F. J. Leeper, F. Blanche and A. R. Battersby
"Biosynthesis of Vitamin B12: Stereochemistry of Transfer of a Hydride Equivalent from NADPH by Precorrin-6x Reductase"
J. Chem. Soc., Chem. Commun.,1992, 306-308.
Full Text
Deuterium labelling with NMR analysis demonstrates that the enzyme precorrin-6x reductase transfers HR from C-4 of the pyridine residue of NADPH as the first step in the sequence leading from precorrin-6x to hydrogenobyrinic acid.
.
.
.
.
.
.
.
.
.
54 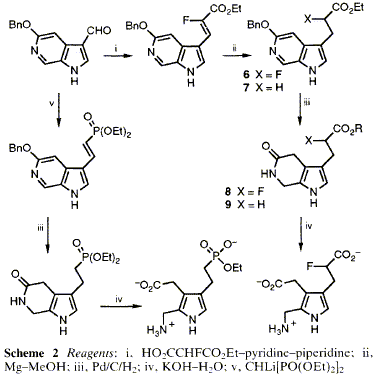 F. J. Leeper and M. Rock
F. J. Leeper and M. Rock
"Modified Substrates for Tetrapyrrole Biosynthesis: Analogues of Porphobilinogen Showing Unusual Inhibition of Porphobilinogen Deaminase"
J. Chem. Soc., Chem. Commun.,1992, 242-244.
Full Text
Syntheses are described of two analogues of porphobilinogen (PBG), a fluoro derivative and a phosphonate, which are the first known unnatural substrates of PBG deaminase; when they act as inhibitors of the reaction of PBG, these compounds produce unusual sigmoidal kinetics, explained by their involvement as poor substrates in the particular mechanism of this enzyme.
.
.
.
.
.
.
.
.
.
55 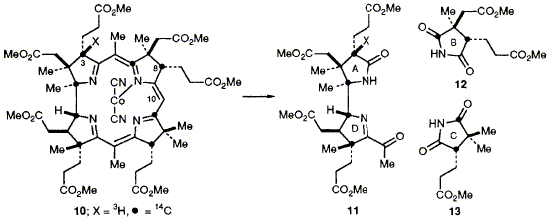 M. Kodera, F. J. Leeper and A. R. Battersby
M. Kodera, F. J. Leeper and A. R. Battersby
"Biosynthesis of Vitamin B12: The Fate of H-8 as Precorrin-2 is Enzymically Converted into Cobyrinic Acid"
J. Chem. Soc., Chem. Commun.,1992, 835-837.
Full Text
The dimethylated B12-Precursor, precorrin-2, is prepared strongly labelled with tritium at 3-H and 8-H to allow proof that the 3-H label is retained where as the 8-H label is lost during the biosynthetic conversion of precorrin-2 into cobyrinic acid.
.
.
.
.
.
.
.
.
.
56 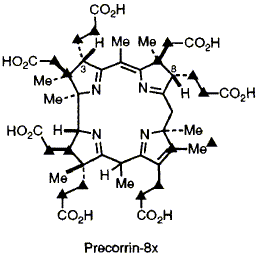 D. Thibaut, F. Kiuchi, L. Debussche, F. Blanche, M. Kodera, F. J. Leeper and A. R. Battersby
D. Thibaut, F. Kiuchi, L. Debussche, F. Blanche, M. Kodera, F. J. Leeper and A. R. Battersby
"Biosynthesis of Vitamin B12: Structural Studies on Precorrin-8x, an Octamethylated Intermediate and the Structure of its Stable Tautomer"
J. Chem. Soc., Chem. Commun.,1992, 982-985.
Full Text
The structure of precorrin-8x, the octamethylated B12 intermediate which follows precorrin-6y in Pseudomonas denitrificans, is largely determined by 13C-labelling and NMR spectroscopy and the structure of its most stable tautomer is established.
.
.
.
.
.
.
.
.
.
57 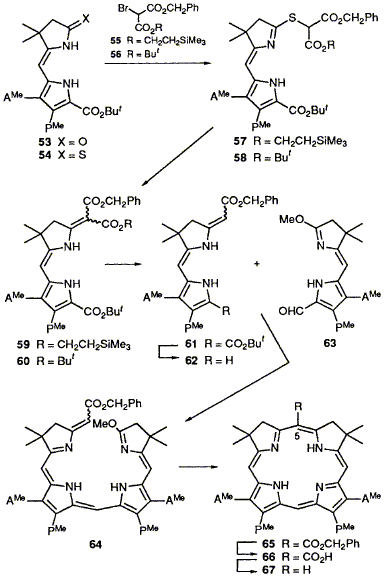 A. R. Battersby, M. H. Block, C. J. R. Fookes, P. J. Harrison, G. B. Henderson and F. J. Leeper
A. R. Battersby, M. H. Block, C. J. R. Fookes, P. J. Harrison, G. B. Henderson and F. J. Leeper
"Synthetic Studies Relevant to Biosynthetic Research on Vitamin B12, Part 10. Construction of the East and West Building Blocks for Synthesis of Isobacteriochlorins"
J. Chem. Soc., Perkin Trans. 1,1992, 2175-2188.
Full Text
Studies with model compounds have led to the development of effective methods for (a) linking the pyrrolic rings to the reduced rings present in the isobacteriochlorin system and (b) for introducing the carbon at C-5 required to complete the macrocycle. In the course of this work, many new pyrrolic systems have been prepared and characterised.
.
.
.
.
.
.
.
.
.
58 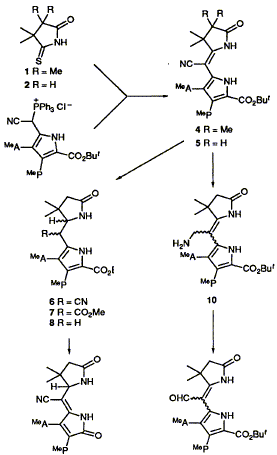 A. R. Battersby, M. H. Block, F. J. Leeper and S. C. Zimmerman
A. R. Battersby, M. H. Block, F. J. Leeper and S. C. Zimmerman
"Synthetic Studies Relevant to Biosynthetic Research on Vitamin B12, Part 11. Modification of the East and West Building Blocks and Study of Different Assembly Methods for Synthesis of Isobacteriochlorins"
J. Chem. Soc., Perkin Trans. 1,1992, 2189-2195.
Full Text
The eastern and western building blocks required for the photochemical route to isobacteriochlorins have been synthesized by a C-C bond-forming step which leaves a cyano group on the bridge carbon. Two methods have been developed to remove this cyano residue which use retro-Mannich reactions to eliminate either an aminomethyl group, resulting from reduction of the nitrile, or the corresponding sulfonamido group. Two effective ways to carry out the final steps of the isobacteriochlorin synthesis have also been developed.
.
.
.
.
.
.
.
.
.
59 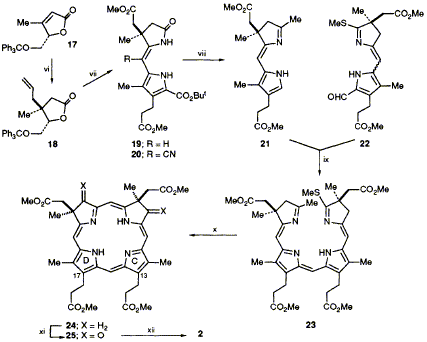 J. Micklefield, R. L. Mackman, C. J. Aucken, M. Beckmann, M. H. Block, F. J. Leeper and A. R. Battersby
J. Micklefield, R. L. Mackman, C. J. Aucken, M. Beckmann, M. H. Block, F. J. Leeper and A. R. Battersby
"A Novel Stereoselective Synthesis of the Macrocycle of Heme d1 that Establishes its Absolute Configuration as 2R,7R"
J. Chem. Soc., Chem. Commun.,1993, 275-277.
Full Text
A novel route to isobacteriochlorins is developed that allows the stereoselective synthesis of the macrocycle of haem d1 and so establishes its absolute configuration as 2R,7R.
.
.
--- Next ten abstracts ----- Previous ten abstracts ---
.
.