

40
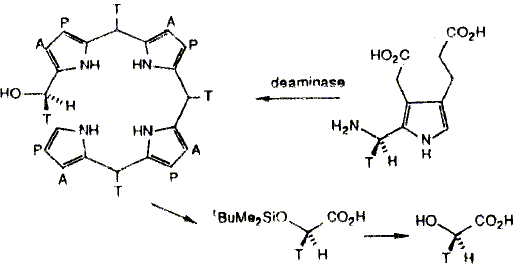
Porphobilinogen (PBG) deaminase catalyses the tetramerization of the monopyrrole PBG to give a linear tetrapyrrole, hydroxymethylbilane (HMB). The stereochemistry of some of the steps in this reaction has been investigated using PBG chirally labelled with deuterium or tritium at the aminomethyl group (C-11). Two different syntheses of the chirally labelled PBG are described and methods for the analysis of the stereochemistry of the products are detailed. These studies showed that the formation of HMB proceeds with overall retention of configuration at the hydroxymethyl carbon. This rules out the release of the azafuIvene intermediate from the enzyme.
.
.
.
.
.
.
.
.
.
.
41
The stereospecifically deuteriated phenyl cyclopropyl carbinol (3b) was synthesized from chiral alcohol (8). Ring-opening to the homoallylic alcohol (4) was shown to proceed with clean inversion of configuration by 1H n.m.r. spectroscopy of the ring-opened product with a chiral shift reagent. The indolizidine alkaloid (1) can be degraded to (3) and this will provide a method to analyse the stereochemistry of its biosynthesis.
.
.
.
.
.
.
.
.
.
.
42
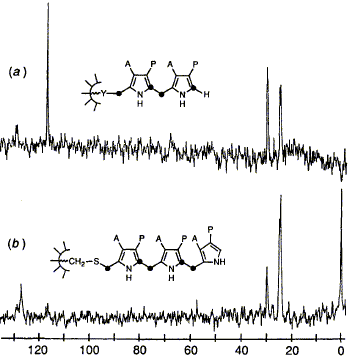
The enzyme hydroxymethyl bilane synt hase constructs the open-chain hydroxymethyl bilane by
assembly of four porphobilinogen units head-to-tail, the first of these being covalently bound to the
enzyme through a group X. The surprising discovery is made that X is a novel dipyrromethane
cofactor constructed from two porphobilinogen units and bound to the protein via the sulphur of
cysteine. This cofactor does not turn over in the catalytic process but acts as an anchor for the
assembly of a hexapyrrole from which the tetrapyrrolic hydroxymethylbilane is cleaved leaving the
dipyrromethane cofactor in place for a further building cycle.
.
.
.
.
.
.
.
.
.
.
43
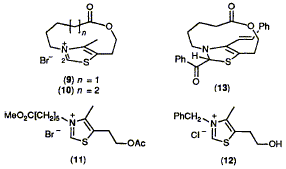
The reactivity of bridged thiazolium salts (9) and (10) has been studied; the model (9) with the shorter bridge is unable to catalyse the benzoin condensation reaction and it was shown that this is due to failure of the
condensation step (5) -> (6), probably owing to the effects of strain.
.
.
.
.
.
.
.
.
.
.
44
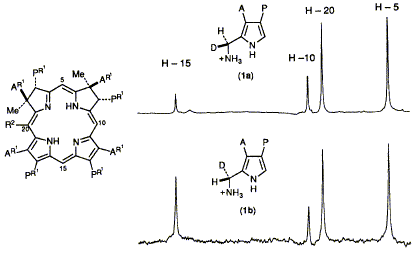
(11S)-[2H1]Porphobilinogen ( 1a) and the (11R)-isomer (1b) are incorporated biosynthetically into precorrin-2 and precorrin-3 which are isolated as the aromatised esters (7) and (9) for 1H NMR studies; the unexpected results are discussed.
.
.
.
.
.
.
.
.
.
.
45
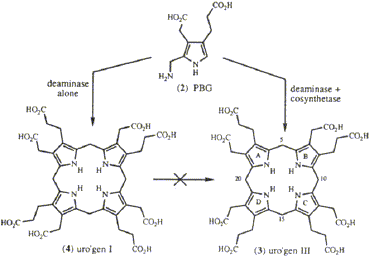
The tetrapyrrolic pigments of life include the hemes,
chlorophylls, vitamin B12, and the light-harvesting bilins
of algae. They are all derived from the same tetrapyrrolic
macrocycle, uroporphyrinogen III (3), which is
generally abbreviated uro’gen III (Scheme I). In this
review we are concerned with the mechanistic aspects
of the biosynthesis of uro’gen III from its monopyrrolic
precursor, porphobilinogen (2, known as PBG), catalyzed
by the enzymes PBG deaminase and uro’gen III
synthase (or cosynthetase).
.
.
.
.
.
.
.
.
.
.
46
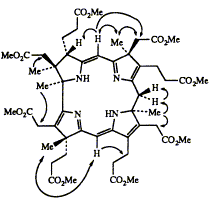
13C-labeled precorrin-6x is biosynthesized by
cell-free protein preparations from Pseudomonas denitrificans
in separate experiments using 5-amino[5-13C]levulinic acid and
the corresponding 5-amino[4-13C]- and 5-amino[3-13Clevulinic
acid-labeled forms in conjunction with S-[methyl-13C]adenosylmethionine for the latter two experiments. These
labeled precorrin-6x samples, as their octamethyl esters, are
studied by a range of NMR techniques. In addition, nuclear
Overhauser effect difference measurements are made on unlabeled
precorrin-6x ester to determine connectivities. The
structure 6a so established for precorrin-6x ester (i) confirms
the results reported in the preceding paper that precorrin-6x
has a ring-contracted macrocycle, still carries the C-12 acetate
residue, and stands at the oxidation level of a dehydrocorrin;
(ii) reveals the unexpected methylation at C-11 not C-12,
leading to a structure with separated chromophores; and (iii)
implies that methyl migration from C-11 to C-12 occurs when
precorrin-6x is converted into hydrogenobyrinic acid. Proposals
for the biosynthesis ofthe corrin macrocycle of hydrogenobyrinic
acid and vitamin B12 are made.
.
.
.
.
.
.
.
.
.
.
47
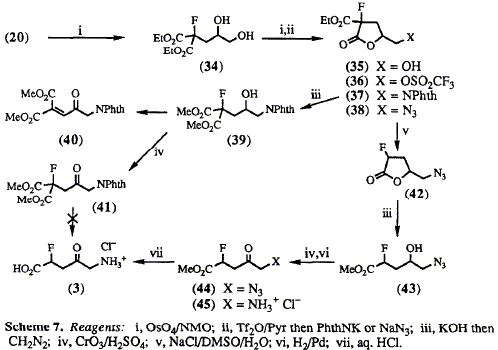
5-Amino-2-fluorolaevulinic acid 3 has been synthesized in high yield
by modification of a new route to 5-aminolaevulinic acid (ALA), involving gamma-lactone
intermediates. Initial studies suggest that 3 is an inhibitor of ALA
dehydratase, an early enzyme of tetrapyrrole biosynthesis.
.
.
.
.
.
.
.
.
.
.
48
We have seen in the preceding chapter that the biosynthetic route to 5-aminolevulinic
acid (ALA) differs in different organisms. However, the remainder of the pathway that is
common to the hemes and the chlorophylls (i.e. until the insertion of the metal ion) is
remarkably consistent throughout the whole of nature. It is this part of the biosynthesis
that forms the main part of the present chapter (Sections I to VI). Also covered are the steps
leading to heme and the linear tetrapyrroles of plants and algae (Sections VII and VIII) and
part of the pathway to chlorophylls (Section IX) and bacteriochlorophylls (Section X).
.
.
.
.
.
.
.
.
.
.
49
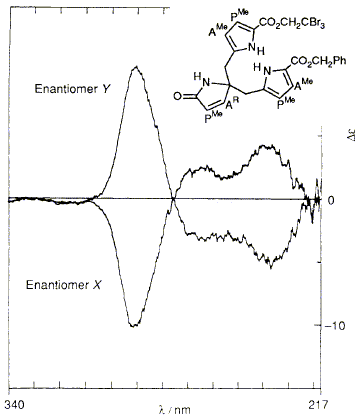
The intermediacy of the spiro-pyrrolenine for biosynthesis of uro'gen III is
supported by synthesis of both enantiomers of the spiro-lactam followed by demonstration
that cosynthetase, the enzyme which forms uro'gen III, is inhibited over twenty
times more strongly by one enantiomer than by the other.
.
.
--- Previous ten abstracts ------ Next ten abstracts ---
.
 F. J. Leeper and A. R. Battersby
F. J. Leeper and A. R. Battersby
"Chirally Tritiated and Deuteriated Compounds in Studies of Biosynthesis"
in Synthesis and Applications of Isotopically Labelled Compounds (Proceedings of the Third International Symposium). 1989, Elsevier, p. 41-46.
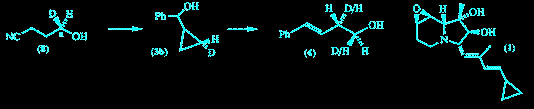
F. J. Leeper and P. Padmanabhan
"Stereospecific Nucleophilic Ring-Opening of a Deuteriated Cyclopropylcarbinol"
Tetrahedron Lett.,1989, 30, 5017-5020.
Full Text
G. J. Hart, A. D. Miller, U. Beifuss, F. J. Leeper and A. R. Battersby
"Biosynthesis of Porphyrins and Related Macrocycles, Part 35. Discovery of a Novel Dipyrrolic Cofactor Essential for the Catalytic Action of Hydroxymethylbilane Synthase (Porphobilinogen Deaminase)"
J. Chem. Soc., Perkin Trans. 1,1990, 1979-1993.
Full Text
F. J. Leeper and D. H. C. Smith
"The Effect of Strain on the Catalytic Reactions of Bridged Thiazolium Salts as Models of Thiamin"
J. Chem. Soc., Chem. Commun.,1990, 961-963.
Full Text
 G. W. Weaver, F. Blanche, D. Thibaut, L. Debussche, F. J. Leeper and A. R. Battersby
G. W. Weaver, F. Blanche, D. Thibaut, L. Debussche, F. J. Leeper and A. R. Battersby
"Biosynthesis of Vitamin B12: Incorporation of 11S-[11-2H]- and 11R-[11-2H]-Porphobilinogen into Sirohydrochlorin and 2,7,20-Trimethylisobacteriochlorin"
J. Chem. Soc., Chem. Commun.,1990, 1125-27.
 A. R. Battersby and F. J. Leeper
A. R. Battersby and F. J. Leeper
"Biosynthesis of the Pigments of Life: Mechanistic Studies on the conversion of Porphobilinogen into Uroporphyrinogen III"
Chem. Rev.,1990, 90, 1261-1274.
Full Text
D. Thibaut, L. Debussche, F. Blanche, F. J. Leeper and A. R. Battersby
"Biosynthesis of Vitamin B12: The Structure of Precorrin-6x Octamethyl Ester"
Proc. Natl. Acad. Sci. USA,1990, 87, 8800-8804.
Full Text
F. J. Leeper and M. Rock
"Synthesis of a Fluorinated Analogue of 5-Aminolaevulinic Acid, a Potential Inhibitor of Porphyrin Biosynthesis"
J. Fluor. Chem.,1991, 51, 381-396.
Full Text
F. J. Leeper
"Intermediate Steps in the Biosynthesis of Chlorophylls"
in Chlorophylls, H. Scheer, Editor. 1991, CRC Press, Boca Raton, p. 407-431.
M. A. Cassidy, N. Crockett, F. J. Leeper and A. R. Battersby
"Synthetic Studies on the Proposed Spiro Intermediate for Biosynthesis of the Natural Porphyrins: The Stereochemical Probe"
J. Chem. Soc., Chem. Commun.,1991, 384-386.