An initial "c" implies the rearragement of the pyrophosphate from C-1 to C-3, necessary for forming a cis-double bond between C-2 and C-3 and cyclising to form a 5-, 6- or 7-membered ring.
A protonation or methylation to form the initial cation is indicated by "H+n,m" or "Me+n,m" where n is the number of the carbon that is protonated and m is the number of the carbon that acquires the positive charge. Otherwise the initial cation is formed by the leaving of the pyrophosphate anion to give the carbocation at the carbon whose number starts the string.
A cyclisation to this cationic carbon is indicated by "-n,m" where n is the number of the carbon that forms the new bond and m is the number of the carbon atom that has the cation after this step.
Cyclisation from an oxygen atom is indicated by "-On" where n is the number of the carbon to which the oxygen is attached.
Nucleophilic attack by a water molecule or the pyrophosphate ion or H- from NADPH is indicated by "-w" or "-OPP" or "-(H-NADP)" respectively.
A Wagner-Meerwein shift of a carbon is indicated by "*n,m" where n is the number of the carbon that forms the new bond and m is the number of the carbon atom that has the cation after this step. If carbon n is already bonded to the carbocation then this is a bond cleavage step that generates a double bond.
A hydride shift is indicated by "*Hn" where n is the number of the carbon atom that did have the H on and which has the cation after this step.
A loss of a proton is indicated by "-Hn" where n is the number of the carbon atom that is deprotonated. Where the proton is transferred direct to another carbon atom (and not to an enzymic residue) this is given as "-Hn+m,p" where n is the atom losing the proton, m is the atom gaining it and p is the atom which ends up with the positive charge.
Where the mechanism proceeds through a neutral intermediate (by loss of H+ or attack of water or OPP) this is indicated by "; ".
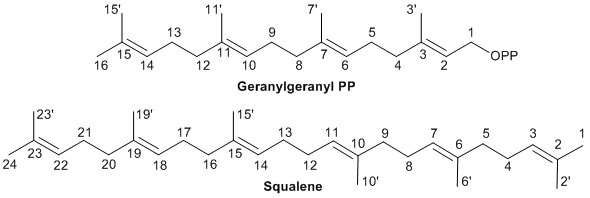
The carbon atom numbering is that of the precursor (e.g. farnesyl PP) and each atom keeps the same number from beginning to end of the mechanism. The numbering used for geranylgeranyl PP and squalene (as examples) is the IUPAC recommended numbering shown here.

Where two prenyl diphosphates react together (e.g. two DMAPP or two FPP molecules) this is indicated in the number of C5 units column (e.g. "1+1" or "3+3") and the atom numbering for the first molecule is in normal type-face while the atom numbering for the second molecule is in italic.